Abstract
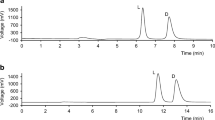
Indirect enantioresolution of 15 primary and secondary amino group containing compounds (amino alcohols, non-protein amino acids, PenA) was done using the reagent (S)-N-(4-Nitrophenoxycarbonyl) phenylalanine methoxyethyl ester [(S)-NIFE] by reversed-phase high-performance liquid chromatography. The diastereomeric derivatives were analyzed under reversed-phase conditions using linear gradient. The detection was at 205 nm and sharp peaks were obtained. The reagent used is comparatively economic than the other derivatizing reagents. Method validation was also done.



Similar content being viewed by others
References
Árki A, Tourwé D, Solymár M, Fülöp F, Armstrong DW, Péter A (2004) High-performance liquid chromatographic separation of stereoisomers of ß-amino acids and a comparison of separation efficiencies on chirobiotic T and TAG columns. Chromatographia 60:S43–S54
Bhushan R, Kumar V (2008a) Indirect enantioseparation of α-amino acids by reversed-phase liquid chromatography using new chiral derivatizing reagents synthesized from s-triazine chloride. J Chromatogr A 1201:35–42
Bhushan R, Kumar V (2008b) Synthesis and application of new chiral variants of Marfey’s reagent for liquid chromatographic separation of the enantiomers of α-amino acids. Acta Chromatogr 20:329–347
Bhushan R, Kumar R (2010) Enantioresolution of dl-penicillamine. Biomed Chromatogr 24:66–92. doi:10.1002/bmc.1355
Bhushan R, Brückner H, Kumar V (2007) Indirect resolution of enantiomers of penicillamine by TLC and HPLC using Marfey’s reagent and its variants. Biomed Chromatogr 21:1064–1068
Bhushan R, Kumar V, Tanwar S (2009) Chromatographic separation of enantiomers of non-protein α-amino acids after derivatization with Marfey’s reagent and its four variants. Amino Acids 36:571–579. doi:10.1007/s00726-008-0135-5
Fujii K, Ikai Y, Mayumi T, Oka H, Suzuki M, Harada K (1997) A nonempirical method using LC/MS for determination of the absolute configuration of constituent amino acids in a peptide: elucidation of limitations of Marfey’s method and of its separation mechanism. Anal Chem 69:3346–3352
Hess S, Gustafson KR, Milanowski DJ, Alvira E, Lipton MA, Pannell LK (2004) Chirality determination of unusual amino acids using precolumn derivatization and liquid chromatographyelectrospray ionization mass spectrometry. J Chromatogr A 1035:211–219
Ito S, Ota A, Yamamoto K, Kawashima Y (1992) Resolution of the enantiomers of thiol compounds by reversed-phase liquid chromatography using chiral derivatization with 2, 3, 4, 6-tetra-O-acetyl-β-d-glucopyranosylisothiocyanate. J Chromatogr 626:187–196
Jin D, Toyo’oka T (1998) Indirect resolution of thiol enantiomers by high-performance liquid chromatography with a fluorescent chiral tagging reagent. Analyst 123:1271–1277
Olajos E, Péter A, Casimir R, Tourwé D (2001) HPLC enantioseparation of phenylalanine analogs by application of (S)-N-(4-nitrophenoxycarbonyl)phenylalanine methoxyethyl ester as a new chiral derivatizing agent. Chromatographia 54:77–82
Péter A, Vékes E, Torok G (2000) Application of (S)-N-(4-nitrophenoxycarbonyl) phenylalanine methoxyethyl ester as a new chiral derivatizing agent for proteinogenic amino acid analysis by high performance liquid chromatography. Chromatographia 52:821–826
Péter A, Vékes E, Toth G, Tourwé D, Borremans F (2002) Application of a new chiral derivatizing agent to the enantioseparation of secondary amino acids. J Chromatogr A 948:283–294
Péter A, Arki A, Vékes E, Tourwé D, Lázár L, Fülöp F, Armstrong DW (2004) Direct and indirect high-performance liquid chromatographic enantioseparation of β-amino acids. J Chromatogr A 1031:171–178
Vékes E, Torok G, Péter A, Sapi J, Tourwé D (2002) Indirect high-performance liquid chromatographic separation of stereoisomers of β-alkyl-substituted amino acids by the application of (S)-N-(4-nitrophenoxycarbonyl)phenylalanine methoxyethyl ester as chiral derivatizing agent. J Chromatogr A 949:125–139
Acknowledgments
The authors are thankful to the Ministry of Human Resources Development, Government of India, New Delhi, for the award of a research assistantship (to C.A.) and to the Alexander von Humboldt Foundation, Bonn, Germany, for donating Knauer HPLC equipment and award of a fellowship (to R.B.)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bhushan, R., Agarwal, C. Application of (S)-N-(4-Nitrophenoxycarbonyl) phenylalanine methoxyethyl ester as a chiral derivatizing reagent for reversed-phase high-performance liquid chromatographic separation of diastereomers of amino alcohols, non-protein amino acids, and PenA. Amino Acids 39, 549–554 (2010). https://doi.org/10.1007/s00726-010-0472-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-010-0472-z