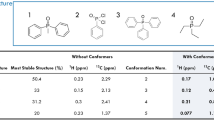
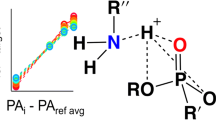
Abstract
Organophosphorus (OP) nerve agents are a group of lethal small molecules. Fieldable detection of nerve agents is an on-going challenge that typically relies on mass spectrometry and infrared spectroscopy but not nuclear magnetic resonance (NMR) spectroscopy because of the portability limitations of superconducting magnets. However, Earth’s field NMR (EFNMR) demonstrates a unique signature space for OP compounds and can be made into a portable detector for OP nerve agents. Here we demonstrate a systematic study to develop the EFNMR signature space of 31 nerve-agent-related OP compounds, including surrogates, simulants, synthetic precursors, decomposition products, pesticides, and threat agents identified by the National Institutes of Health. The EFNMR spectral signatures are a diagnostic fingerprint of the molecular structure, and this study establishes the structure–signature relationships of this relatively unexplored signature space. The results indicate that EFNMR is a powerful analytical capability to distinguish and identify the unique structure of OP compounds, including nerve agents. While aimed at detection of nerve agents, this study also lays the foundations of using EFNMR for detection of any OP compound in the laboratory or in the field.









Similar content being viewed by others
Availability of Data and Materials
Data for the J-couplings reported in this article that were used for the simulations of the nerve agent JCS are available upon request from the documentation counter at the OPCW Headquarters building. All other data supporting the findings of this study are available within the paper and its Supplementary Information files. Should any raw data files be needed in another format, they are available from the corresponding author upon reasonable request.
References
S. Chauhan, S. Chauhan, R. D’Cruz, S. Faruqi, K.K. Singh, S. Varma, M. Singh, V. Karthik, Chemical warfare agents. Environ. Toxicol. Pharmacol. 26, 113–122 (2008)
R. Fatz, Implementation guidance policy for new airborne exposure limits for GB, GA, GD, GF, VX, H, HD and HT (Memorandum. US Department of the Army, Office of Assistant Secretary, Installations and Environment, Washington DC, 2004)
M. Moshiri, E. Darchini-Maragheh, M. Balali-Mood, Advances in toxicology and medical treatment of chemical warfare nerve agents. Daru 20, 81 (2012)
F. Worek, M. Koller, H. Thiermann, L. Szinicz, Diagnostic aspects of organophosphate poisoning. Toxicology 214, 182–189 (2005)
O. Nosseir, C. Hadad, Chemical Warfare Agents and Treatments (American Chemical Society, 2021)
S. Mogl, Sampling and analysis in the Chemical Weapons convention and the OPCW mobile laboratory. In: Mesilaakso M (Ed.), Chemical Weapons Convention Chemicals and Analysis, Sample Collection Preparation and Analytical Methods, Wiley, Hoboken, NJ, Wiley Online Library, pp 7–31 (2005)
Z. Witkiewicz, S. Neffe, E. Sliwka, J. Quagliano, Analysis of the precursors, simulants and degradation products of chemical warfare agents. J Crit. Rev. Analyt. Chem. 48, 337–371 (2018)
A. Abragam, The Principles of Nuclear Magnetism, Oxford university press, 1961.
M.J. Duer, Introduction to Solid-State NMR Spectroscopy (Blackwell Oxford, 2004)
C.P. Slichter, Principles of magnetic resonance (1990)
H. Koskela, B. Andelkovic, NMR chemical shift and J coupling parameterization and quantum mechanical reference spectrum simulation for selected nerve agent degradation products in aqueous conditions. Magn. Reson. Chem. 55, 917–927 (2017)
D.C. Kaseman, M.W. Malone, A. Tondreau, M.A. Espy, R.F. Williams, Quantitation of nuclear magnetic resonance spectra at Earth’s magnetic field. Anal. Chem. 93, 15349–15357 (2021)
S. Appelt, F. Haesing, H. Kuhn, B. Blumich, Phenomena in J-coupled nuclear magnetic resonance spectroscopy in low magnetic fields. Phys. Rev. A 76, 023420 (2007)
S. Appelt, F. Hasing, H. Kuhn, U. Sieling, B. Blumich, Analysis of molecular structures by homo- and hetero-nuclear J-coupled NMR in ultra-low field. Chem. Phys. Lett. 440, 308–312 (2007)
S. Appelt, F. Hasing, U. Sieling, A. Gordji-Nejad, S. Gloggler, B. Blumich, Paths from weak to strong coupling in NMR. Phys. Rev. A 81, 023420 (2010)
S. Appelt, H. Kuhn, F. Hasing, B. Blumich, Chemical analysis by ultrahigh-resolution nuclear magnetic resonance in the Earths magnetic field. NatPh 2, 105–109 (2006)
B. Blumich, F. Casanova, S. Appelt, NMR at low magnetic fields. Chem. Phys. Lett. 477, 231–240 (2009)
J. Colell, P. Turschmann, S. Gloggler, P. Schleker, T. Theis, M. Ledbetter, D. Budker, A. Pines, B. Blumich, S. Appelt, Fundamental aspects of parahydrogen enhanced low-field nuclear magnetic resonance, Phys. Rev. Lett., 110, 137602-1–137602-5 (2013)
S. Gloggler, B. Blumich, S. Appelt, H. Heise, S. Matthews, NMR spectroscopy for chemical analysis at low magnetic fields, modern NMR. Methodology 335, 1–22 (2013)
P. Callaghan, A. Coy, R. Dykstra, C. Eccles, M. Halse, M. Hunter, O. Mercier, J. Robinson, New Zealand developments in earth’s field NMR. Appl. Magn. Reson. 32, 63–74 (2007). https://doi.org/10.1007/s00723-007-0012-5
M.E. Halse, P.T. Callaghan, Terrestrial magnetic field NMR: recent advances. In: eMagRes
M.E. Halse, P.T. Callaghan, A dynamic nuclear polarization strategy for multi-dimensional Earth’s field NMR spectroscopy. J. Magn. Reson. 195, 162–168 (2008)
M.E. Halse, P.T. Callaghan, B.C. Feland, R.E. Wasylishen, Quantitative analysis of Earth’s field NMR spectra of strongly-coupled heteronuclear systems. J. Magn. Reson. 200, 88–94 (2009)
M.E. Halse, A. Coy, R. Dykstra, C. Eccles, M. Hunter, R. Ward, P.T. Callaghan, A practical and flexible implementation of 3D MRI in the Earth’s magnetic field. J. Magn. Reson. 182, 75–83 (2006)
F. Hill-Casey, A. Sakho, A. Mohammed, M. Rossetto, F. Ahwal, S.B. Duckett, R.O. John, P.M. Richardson, R. Virgo, M.E. Halse, In Situ SABRE Hyperpolarization with Earth’s Field NMR Detection. Molecules 24, 4126 (2019)
P.M. Richardson, R.O. John, A.J. Parrott, P.J. Rayner, W. Iali, A. Nordon, M.E. Halse, S.B. Duckett, Quantification of hyperpolarisation efficiency in SABRE and SABRE-relay enhanced NMR spectroscopy. Phys. Chem. Chem. Phys. 20, 26362–26371 (2018)
R. McDermott, A.H. Trabesinger, M. Muck, E.L. Hahn, A. Pines, J. Clarke, Liquid-state NMR and scalar couplings in microtesla magnetic fields. Science 295, 2247 (2002)
G.J. Béné, Nuclear magnetism of liquid systems in the Earth field range. PhR 58, 213–267 (1980)
S. Appelt, F. Hasing, H. Kuhn, J. Perlo, B. Blumich, Mobile high resolution xenon nuclear magnetic resonance spectroscopy in the earth’s magnetic field. Phys. Rev. Lett. 94, 197602 (2005)
D.C. Kaseman, M.T. Janicke, R.K. Frankle, T. Nelson, G. Angles-Tamayo, R.J. Batrice, P.E. Magnelind, M.A. Espy, R.F. Williams, Chemical analysis of fluorobenzenes via multinuclear detection in the strong heteronuclear J-coupling regime. Appl. Sci. 10, 3836 (2020)
J. Bernarding, G. Buntkowsky, S. Macholl, S. Hartwig, M. Burghoff, L. Trahms, J-Coupling nuclear magnetic resonance spectroscopy of liquids in nT fields. J. Am. Chem. Soc. 128, 714–715 (2006)
A.H. Trabesinger, R. McDermott, S. Lee, M. Mück, J. Clarke, A. Pines, SQUID-detected liquid state NMR in microtesla fields. J. Phys. Chem. A 108, 957–963 (2004)
S.-H. Liao, M.-J. Chen, H.-C. Yang, S.-Y. Lee, H.-H. Chen, H.-E. Horng, S.-Y. Yang, A study of J-coupling spectroscopy using the Earth’s field nuclear magnetic resonance inside a laboratory. Rev. Sci. Instrum. 81, 104104 (2010)
M. Packard, R. Varian, Proton gyromagnetic ratio. Phys. Rev 93, 417–418 (1954)
C.S. Kang, K. Kim, S.-J. Lee, S.-M. Hwang, J.-M. Kim, K.K. Yu, H. Kwon, S.K. Lee, Y.-H. Lee, Application of the double relaxation oscillation superconducting quantum interference device sensor to micro-tesla 1H nuclear magnetic resonance experiments. J. Appl. Phys. 110, 053906 (2011)
S.-H. Liao, H.-C. Yang, H.-E. Horng, S.Y. Yang, H.H. Chen, D.W. Hwang, L.-P. Hwang, SensitiveJ-coupling spectroscopy using high-Tcsuperconducting quantum interference devices in magnetic fields as low as microteslas. Supercond. Sci. Technol. 22, 045008 (2009)
G. Bevilacqua, V. Biancalana, A.B.-A. Baranga, Y. Dancheva, C. Rossi, Microtesla NMR J-coupling spectroscopy with an unshielded atomic magnetometer. J. Magn. Reson. 263, 65–70 (2016)
P. Bergstrom Mann, S. Clark, S.T. Cahill, C.D. Campbell, M.T. Harris, S. Hibble, T. To, A. Worrall, M. Stewart, Implementation of earth’s field NMR spectroscopy in an undergraduate chemistry laboratory. J. Chem. Educ. 96, 2326–2332 (2019)
D.C. Kaseman, P.E. Magnelind, S.W. Paisner, J.L. Yoder, M. Alvarez, A.V. Urbaitis, M.T. Janicke, P. Nath, M.A. Espy, R.F. Williams, Design and implementation of a J-coupled spectrometer for multidimensional structure and relaxation detection at low magnetic fields. Rev. Sci. Instrum. 91, 054103 (2020)
S.H. Liao, H.H. Chen, Y.S. Deng, M.W. Wang, K.L. Chen, C.W. Liu, C.I. Liu, H.C. Yang, H.E. Horng, J.J. Chieh, S.Y. Yang, Microtesla NMR and high resolution MR imaging using high-Tc SQUIDs. ITAS 23, 1602404–1602404 (2013)
V.I. Chizhik, P.A. Kupriyanov, G.V. Mozzhukhin, NMR in magnetic field of the earth: pre-polarization of nuclei with alternating magnetic field. Appl. Magn. Reson. 45, 641–651 (2014)
R. Wärme, L. Juhlin, A new microscale method for the conversion of phosphorus oxyacids to their fluorinated analogues, using cyanuric fluoride in solution and on solid support. Phosphorus Sulfur Silicon Relat. Elem. 185, 2402–2408 (2010)
J.G. Riess, R. Bender, J.-C. Elkaim, Ligandenaustauschreaktionen von Fluoratomen mit Chloratomen bei Phosphorverbindungen der Koordinationszahl 3 und 4. Z. Anorg. Allg. Chem. 391, 60–68 (1972)
H.J. Hogben, M. Krzystyniak, G.T.P. Charnock, P.J. Hore, I. Kuprov, Spinach—a software library for simulation of spin dynamics in large spin systems. J. Magn. Reson. 208, 179–194 (2011)
D.C. Kaseman, R.J. Batrice, R.F. Williams, Detection of natural abundance 13C J-couplings at Earth’s magnetic field for spin system differentiation of small organic molecules. J. Magn. Reson. 342, 107272 (2022)
D.I. Hoult, Fast recovery, high sensitivity NMR probe and preamplifier for low frequencies. Rev. Sci. Instrum. 50, 193–200 (1979)
J.L. Vicini, P.K. Jensen, B.M. Young, J.T. Swarthout, Residues of glyphosate in food and dietary exposure. Comprehensive Rev. Food Sci. Food Saf. 20, 5226–5257 (2021)
X. Wang, Q. Lu, J. Guo, I. Ares, M. Martínez, M.-R. Martínez-Larrañaga, X. Wang, A. Anadón, M.-A. Martínez, Oxidative stress and metabolism: a mechanistic insight for glyphosate toxicology. Annu. Rev. Pharmacool. Toxicol. 62, 617–639 (2022)
J. de Wittlaan, OPCW central analytical database, in Organisation for the Prohibition of Chemical Weapons. ed. by OftPoC. Weapons (The Hague, 2019)
N. Guide, Guide for the selection of chemical and biological decontamination equipment for emergency first responders, (2001)
K. Kim, O.G. Tsay, D.A. Atwood, D.G. Churchill, Destruction and detection of chemical warfare agents. Chem. Rev. 111, 5345–5403 (2011)
Acknowledgements
This work is released for publication in accordance with Los Alamos National Laboratory (LANL) LA-UR-22-26916 by Triad National Security, LLC (Los Alamos, NM, USA) operator of the Los Alamos National Laboratory under contract no. 89233218CNA000001 with the U.S. Department of Energy.
Funding
This research was funded by LANL Laboratory Directed Research and Development Projects # 20170048DR, #20210827ER, and # 20210679ECR.
Author information
Authors and Affiliations
Contributions
DCK: conceptualization, methodology, investigation, formal analysis, writing—original draft, visualization, funding acquisition. PEM: conceptualization, methodology, validation, formal analysis, investigation, writing—review and editing. MTJ: conceptualization, methodology, formal analysis, investigation, writing—review and editing. MA: investigation, AT: formal analysis, investigation, resources. SW: conceptualization, formal analysis, investigation, writing—review and editing. RF: validation, investigation. RJB: methodology, resources, writing—review and editing. JLY: methodology, resources, writing—review and editing. AVU: methodology, resources. ME: conceptualization, investigation, supervision, funding acquisition. RFW: conceptualization, investigation, writing—review and editing, supervision, project administration.
Corresponding author
Ethics declarations
Conflict of Interest
Robert F. Williams, Michelle A. Espy, Derrick C. Kaseman, Jacob Luther Yoder, Per Erik Magnelind, Algis V. Urbaitis, Michael Timothy Janicke, and Scarlett Widgeon Paisner have patent #US11525879B2 issued to Triad National Security LLC.
Ethical Approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Prepared for Applied Magnetic Resonance issue on the occasion of Bernhard Blümich’s 70th birthday.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kaseman, D.C., Magnelind, P.E., Janicke, M.T. et al. Earth’s Field NMR for Organophosphate Chemical Warfare Agent Detection. Appl Magn Reson 54, 1297–1320 (2023). https://doi.org/10.1007/s00723-023-01565-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00723-023-01565-4