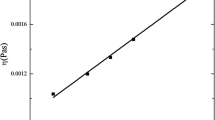
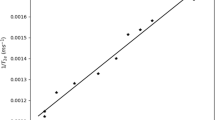
Abstract
The viscosity measurements are of clinical significance for evaluation of the potential pathological conditions of biological lubricants, such as synovial fluids of joints, and for formulation and characterization of peptide- and protein-based biotherapeutics. Due to inherent potential therapeutic activity, protein drugs have proven to be one of the most efficient therapeutic agents in treatment of several life-threatening disorders, such as diabetes and autoimmune diseases. However, home-use applications for treating chronic inflammatory diseases, such as diabetes and rheumatoid arthritis, necessitate the development of high-concentration insulin and monoclonal antibodies formulations for patient self-administration. High protein concentrations can affect viscosity of the corresponding drug solutions complicating their manufacture and administration. The measurements of the viscosity of new insulin analogs and monoclonal antibodies solutions under development is of practical importance to avoid unwanted highly viscous, and therefore, painful for injection drug formulations. Recently, we have demonstrated capability of the electron paramagnetic resonance (EPR) viscometry using viscosity-sensitive 13C-labeled trityl spin probe (13C1-dFT) to report the viscosity of human blood, and interstitial fluids measured in various organs in mice ex vivo and in anesthetized mice, in vivo. In the present work, we demonstrate utility of the EPR viscometry using 13C1-dFT to measure microviscosity of commercial insulin samples, antibodies solution, and human synovial fluids using small microliter volume samples (5–50 µL). This viscometry analysis approach provides useful tool to control formulations and administration of new biopharmaceuticals, and for evaluation of the state of synovial fluids of importance for clinical applications.





Similar content being viewed by others
Availability of Data and Materials
Data from this study can be obtained upon request to VVK (valery.khramtsov@hsc.wvu.edu) or MV (murugesan.velayutham@hsc.wvu.edu).
References
M. Poncelet, B. Driesschaert, Angew. Chem. Int. Ed. 59(38), 16451–16454 (2020)
P.D. Morse 2nd., D.M. Lusczakoski, D.A. Simpson, Biochemistry 18(22), 5021–5029 (1979)
A. Clark, J. Sedhom, H. Elajaili, G.R. Eaton, S.S. Eaton, Concepts Magn. Reson. Part A 45A, e21423 (2016)
H.J. Halpern, G.V.R. Chandramouli, C. Yu, M. Peric, E.D. Barth, B.A. Teicher, D. Grdina, Magn. Reson. Chem. 33, S147–S153 (1995)
J.H. Ardenkjær-Larsen, H. Laursen, I. Leunbach, G. Ehnholm, L.-G. Wistrand, J.S. Petersson, K. Golman, J. Magn. Reson. 133, 1–12 (1998)
N. Kocherginsky, H.M. Swartz, Nitroxide Spin Labels. Reactions in Biology and Chemistry (CRC Press, Boca Raton, 1995)
M. Velayutham, M. Poncelet, T.D. Eubank, B. Driesschaert, V.V. Khramtsov, Molecules 26(9), 2781 (2021)
M.E. Blewis, G.E. Nugent-Derfus, T.A. Schmidt, B.L. Schumacher, R.L. Sah, Eur. Cells Mater. 13, 26–38 (2007)
E. Januzzi, T.C.A. Cunha, G. Silva, B.D.M. Souza, A.S.B. Duarte, M.R.S. Zanini, A.M. Andrade, A.R. Pedrosa, A.L.N. Custodio, M.A.A. Castro, Sci. Rep. 12(1), 17976 (2022)
M. Cicognani, S. Rossi, G. Vecchi, A.M. Giori, F. Ferrari, Pharmaceutics 12(7), 681 (2020)
J. Jezek, M. Rides, B. Derham, J. Moore, E. Cerasoli, R. Simler, B. Perez-Ramirez, Adv. Drug Deliv. Rev. 63(13), 1107–1117 (2011)
T. Hong, K. Iwashita, K. Shiraki, Curr. Protein Pept. Sci. 19(8), 746–758 (2018)
S.S. Virk, P.T. Underhill, Mol. Pharm. 19(11), 4233–4240 (2022)
S.J. Shire, Z. Shahrokh, J. Liu, J. Pharm. Sci. 93(6), 1390–1402 (2004)
M. Muttenthaler, G.E. King, D.J. Adams, P.E. Alewood, Nat. Rev. Drug Discov. 20(4), 309–325 (2021)
C.C. Hanna, Y.O. Hermant, P.W.R. Harris, M.A. Brimble, Acc. Chem. Res. 54(8), 1878–1890 (2021)
E.K. Sims, A.L.J. Carr, R.A. Oram, L.A. DiMeglio, C. Evans-Molina, Nat. Med. 27(7), 1154–1164 (2021)
A. Moran-Thomas, N. Engl. J .Med. 385(4), 293–295 (2021)
R. Cheng, N. Taleb, M. Stainforth-Dubois, R. Rabasa-Lhoret, Am. J. Physiol. Endocrinol. Metab. 320(5), E886–E890 (2021)
M.B. Gelb, H.D. Maynard, Macromol. Mater. Eng. 306(9), 2100197 (2021)
P. Gupta, E.K. Makowski, S. Kumar, Y.L. Zhang, J.M. Scheer, P.M. Tessier, Mol. Pharm. 19(3), 775–787 (2022)
H. Bachelez, S.E. Choon, S. Marrakchi, A.D. Burden, T.F. Tsai, A. Morita, A.A. Navarini, M. Zheng, J. Xu, H. Turki, M.J. Anadkat, S. Rajeswari, H. Hua, S.D. Vulcu, D. Hall, K. Tetzlaff, C. Thoma, M.G. Lebwohl, E.T. Investigators, N. Engl. J. Med. 385(26), 2431–2440 (2021)
S. Edavettal, P. Cejudo-Martin, B. Dasgupta, D. Yang, M.D. Buschman, D. Domingo, K. Van Kolen, P. Jaiprasat, R. Gordon, K. Schutsky, B. Geist, N. Taylor, C.H. Soubrane, E. Van Der Helm, A. LaCombe, Z. Ainekulu, E. Lacy, J. Aligo, J. Ho, Y. He, P.F. Lebowitz, J.T. Patterson, J.M. Scheer, S. Singh, Med. 3(12), 860-882e15 (2022)
H. Li, T. Buck, M. Zandonatti, J. Yin, A. Moon-Walker, J. Fang, A. Koval, M.L. Heinrich, M.M. Rowland, R. Diaz Avalos, S.L. Schendel, D. Parekh, D. Zyla, A. Enriquez, S. Harkins, B. Sullivan, V. Smith, O. Chukwudozie, R. Watanabe, J.E. Robinson, R.F. Garry, L.M. Branco, K.M. Hastie, E.O. Saphire, Sci. Transl. Med. 14(668), eabq0991 (2022)
R.N. Amaria, M. Postow, E.M. Burton, M.T. Tetzlaff, M.I. Ross, C. Torres-Cabala, I.C. Glitza, F. Duan, D.R. Milton, K. Busam, L. Simpson, J.L. McQuade, M.K. Wong, J.E. Gershenwald, J.E. Lee, R.P. Goepfert, E.Z. Keung, S.B. Fisher, A. Betof-Warner, A.N. Shoushtari, M. Callahan, D. Coit, E.K. Bartlett, D. Bello, P. Momtaz, C. Nicholas, A. Gu, X. Zhang, B.R. Korivi, M. Patnana, S.P. Patel, A. Diab, A. Lucci, V.G. Prieto, M.A. Davies, J.P. Allison, P. Sharma, J.A. Wargo, C. Ariyan, H.A. Tawbi, Nature 611(7934), 155–160 (2022)
H. Zhang, P.A. Dalby, Mol. Pharm. 19(11), 4098–4110 (2022)
P.J. Carter, G.A. Lazar, Nat. Rev. Drug Discov. 17(3), 197–223 (2018)
R.L. Wu, A.H. Idris, N.M. Berkowitz, M. Happe, M.R. Gaudinski, C. Buettner, L. Strom, S.F. Awan, L.A. Holman, F. Mendoza, I.J. Gordon, Z. Hu, A. Campos Chagas, L.T. Wang, L. Da Silva Pereira, J.R. Francica, N.K. Kisalu, B.J. Flynn, W. Shi, W.P. Kong, S. O’Connell, S.H. Plummer, A. Beck, A. McDermott, S.R. Narpala, L. Serebryannyy, M. Castro, R. Silva, M. Imam, I. Pittman, S.P. Hickman, A.J. McDougal, A.E. Lukoskie, J.R. Murphy, J.G. Gall, K. Carlton, P. Morgan, E. Seo, J.A. Stein, S. Vazquez, S. Telscher, E.V. Capparelli, E.E. Coates, J.R. Mascola, J.E. Ledgerwood, L.K. Dropulic, R.A. Seder, V.R.C.S. Team, N. Engl. J. Med. 387(5), 397–407 (2022)
D. Focosi, S. McConnell, A. Casadevall, E. Cappello, G. Valdiserra, M. Tuccori, Lancet Infect. Dis. 22(11), e311–e326 (2022)
S. Patel, B. Saxena, P. Mehta, Heliyon 7(2), e06158 (2021)
G. Walsh, E. Walsh, Nat. Biotechnol. 40(12), 1722–1760 (2022)
L. Ayalew, P. Chan, Z. Hu, A. Shen, E. Duenas, W. Kirschbrown, A.J. Schick 3rd., Y. Chen, M.T. Kim, Mol. Pharm. 19(11), 4043–4054 (2022)
J. Parvizi, T.L. Tan, K. Goswami, C. Higuera, C. Della Valle, A.F. Chen, N. Shohat, J. Arthroplast. 33(5), 1309–1314 (2018)
S. Stoll, A. Schweiger, J. Magn. Reson. 178(1), 42–55 (2006)
A.C. Anselmo, Y. Gokarn, S. Mitragotri, Nat. Rev. Drug Discov. 18(1), 19–40 (2019)
R.A. Lewus, N.E. Levy, A.M. Lenhoff, S.I. Sandler, Biotechnol. Progr. 31(1), 268–276 (2015)
G.D.O. Lowe, J.C. Barbenel, in Clinical Blood Rheology. ed. by G.D.O. Lowe (CRC Press, Boca Raton, 1988), pp.12–44
W.H. Reinhart, S.J. Danoff, S. Usami, S. Chien, J. Lab. Clin. Med. 104(6), 921–931 (1984)
M.A. Haidekker, A.G. Tsai, T. Brady, H.Y. Stevens, J.A. Frangos, E. Theodorakis, M. Intaglietta, Am. J. Physiol. Heart Circ. Physiol. 282(5), H1609–H1614 (2002)
A. Vysniauskas, I. Lopez-Duarte, N. Duchemin, T.T. Vu, Y. Wu, E.M. Budynina, Y.A. Volkova, E. Pena Cabrera, D.E. Ramirez-Ornelas, M.K. Kuimova, Phys. Chem. Chem. Phys. 19(37), 25252–25259 (2017)
M. Poncelet, T. Ngendahimana, T.D. Gluth, E.H. Hoblitzell, T.D. Eubank, G.R. Eaton, S.S. Eaton, B. Driesschaert, Analyst. 147(24), 5643–5648 (2022)
Funding
This work was supported by the NIH grants EB023990 (BD), EB028553 (BD), GM143595 (BD), EB32321 (BD), CA194013 (VVK) and CA192064 (VVK).
Author information
Authors and Affiliations
Contributions
MV performed EPR studies; MV and VVK wrote the manuscript; MB and BD synthesized the probe; JAP, JTK and MJD provided biological samples. All authors reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
All authors declare no potential conflict of interest, financial or non-financial, relevant to this work.
Ethical Approval
Collection of Human Synovial Fluids (HSF): all subjects (n = 7) was conducted in accordance with the Declaration of Helsinki and approved Institutional Review Board protocol, #1709745853. All subjects gave informed consent for inclusion in the study and consent to publish.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Velayutham, M., Poncelet, M., Perini, J.A. et al. EPR Viscometric Measurements Using a 13C-Labeled Triarylmethyl Radical in Protein-Based Biotherapeutics and Human Synovial Fluids. Appl Magn Reson 54, 779–791 (2023). https://doi.org/10.1007/s00723-023-01556-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00723-023-01556-5