Abstract
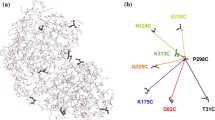
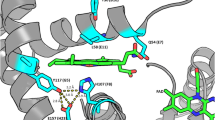
[FeFe]-hydrogenases catalyze the reversible interconversion of protons to molecular hydrogen (H2) at an active site called H-cluster. The maturation pathway of these enzymes is a complex process involving three proteins, HydE, HydF and HydG. The maturase protein HydF has been suggested to interact with HydE and HydG and to be the transferase that shuttles the complete H-cluster to the hydrogenase; however, the exact molecular mechanism driving this translocation remains unclear. HydF is constituted by three different domains: a N-terminal GTP-binding domain, a dimerization domain and a C-terminal [4Fe4S] cluster-binding domain. To investigate possible conformational changes induced by the GTP binding in the N-terminal domain, we have expressed, in Escherichia coli, a recombinant HydF protein from Thermotoga neapolitana including the GTP-binding domain only. Site-directed mutants were designed in which the native residues were substituted by cysteines and subsequently spin labeled with the nitroxide MTSSL. CW-EPR was used to study the local mobility of the nitroxides at each site, and double spin-labeled mutants have been investigated by PELDOR spectroscopy. We found that the binding of the nucleotide does not induce large conformational effects within the isolated GTP domain, at least at the level of the elements investigated in this work. However, small changes in the distance between spin labels were observed which might reflect diffuse structural rearrangements. We suggest that the variations following the GTP binding could affect the dimer form adopted by the whole HydF protein in solution and, as a consequence, the interactions with the other maturases.





Similar content being viewed by others
References
P.M. Vignais, B. Billoud, Chem. Rev. 107, 4206–4272 (2007)
J.W. Peters, W.N. Lanzilotta, B.J. Lemon, L.C. Seefeldt, Science 282, 1853–1858 (1998)
Y. Nicolet, C. Piras, P. Legrand, C.E. Hatchikian, J.C. Fontecilla-Camps, Structure 7, 13–23 (1999)
W. Lubitz, H. Ogata, O. Rüdiger, E. Reijerse, Chem. Rev. 114, 4081–4148 (2014)
E.M. Shepard, F. Mus, J.N. Betz, A.S. Byer, B.R. Duffus, J.W. Peters, J.B. Broderick, Biochemistry 53, 4090–4104 (2014)
J.W. Peters, J.B. Broderick, Annu. Rev. Biochem. 81, 429–450 (2012)
M.C. Posewitz, P.W. King, S.L. Smolinski, L. Zhang, M. Seibert, M.L. Ghirardi, J. Biol. Chem. 279, 25711–25720 (2004)
J.K. Ruback, X. Brazzolotto, J. Gaillard, M. Fontecave, FEBS Lett. 579, 5055–5060 (2005)
X. Brazzolotto, J.K. Rubach, J. Gaillard, S. Gambarelli, M. Atta, M. Fontecave, J. Biol. Chem. 281, 769–774 (2006)
S.E. McGlynn, E.M. Shepard, M.A. Winslow, A.V. Naumov, K.S. Duschene, M.C. Posewitz, W.E. Broderick, J.B. Broderick, J.W. Peters, FEBS Lett. 582, 2183–2187 (2008)
D.W. Mulder, E.S. Boyd, R. Sarma, R.K. Lange, J.A. Endrizzi, J.B. Broderick, J.W. Peters, Nature 465, 248–251 (2010)
I. Czech, S. Stripp, O. Sanganas, N. Leidel, T. Happe, M. Haumann, FEBS Lett. 585, 225–230 (2011)
G. Berggren, A. Adamska, C. Lambertz, T.R. Simmons, J. Esselborn, M. Atta, S. Gambarelli, J.M. Mouesca, E. Reijerse, W. Lubitz, T. Happe, V. Artero, M. Fontecave, Nature 499, 66–69 (2013)
J. Esselborn, C. Lambertz, A. Adamska-Venkatesh, T. Simmons, G. Berggren, J. Noth, J. Siebel, A. Hemschemeier, V. Artero, E. Reijerse, M. Fontecave, W. Lubitz, T. Happe, Nat. Chem. Biol. 9, 607–609 (2013)
J.M. Kuchenreuther, W.K. Myers, D.L.M. Suess, T.A. Stich, V. Pelmenschikov, S.A. Shiigi, S.P. Cramer, J.R. Swartz, R.D. Britt, S.J. George, Science 343, 424–427 (2014)
J.D. Lawrence, H.X. Li, T.B. Rauchfuss, M. Benard, M.M. Rohmer, Angew. Chem. Int. Ed. 40, 1768–1771 (2001)
P.W. King, M.C. Posewitz, M.L. Ghirardi, M. Seibert, J. Bacteriol. 188, 2163–2172 (2006)
L. Cendron, P. Berto, S. D’Adamo, F. Vallese, C. Govoni, M.C. Posewitz, G.M. Giacometti, P. Costantini, G. Zanotti, J. Biol. Chem. 286, 43944–43950 (2011)
P. Berto, M. Di Valentin, L. Cendron, F. Vallese, M. Albertini, E. Salvadori, G.M. Giacometti, D. Carbonera, P. Costantini, BBA-Bioenergetics 1817, 2149–2157 (2012)
E.M. Shepard, S.E. McGlynn, A.L. Bueling, C.S. Grady-Smith, S.J. George, M.A. Winslow, S.P. Cramer, J.W. Peters, J.B. Broderick, Proc. Natl. Acad. Sci. USA 107, 10448–10453 (2010)
F. Vallese, P. Berto, M. Ruzzene, L. Cendron, S. Sarno, E. De Rosa, G.M. Giacometti, P. Costantini, J. Biol. Chem. 287, 36544–36555 (2012)
A. Scrima, A. Wittinghofer, EMBO J. 25, 2940–2951 (2006)
M.J. Ryle, W.N. Lanzilotta, L.C. Seefeldt, Biochemistry 35, 9424–9434 (1996)
S.B. Jang, M.S. Jeong, L.C. Seefeldt, J.W. Peters, J. Biol. Inorg. Chem. 9, 1028–1033 (2004)
H.J. Chiu, J.W. Peters, W.N. Lanzilotta, M.J. Ryle, L.C. Seefeldt, J.B. Howard, D.C. Rees, Biochemistry 40, 641–650 (2001)
G. Jeschke, M. Pannier, H.W. Spiess, in Distance Measurements in Biological Systems, vol. 19, ed. by L.J. Berliner, S.S. Eaton, G.R. Eaton (Kluwer Academic, New York, 2000), pp. 493–512
O. Schiemann, T.F. Prisner, Quart. Rev. Biophys. 40, 1 (2007)
Y.D. Tsvetkov, A.D. Milov, A.G. Maryasov, Russ. Chem. Rev. 77, 487 (2008)
G. Jeschke, Annu. Rev. Phys. Chem. 63, 419 (2012)
S. Stoll, A. Schweiger, J. Magn. Reson. 178, 42–55 (2006)
Y. Polyhach, E. Bordignon, G. Jeschke, Phys. Chem. Chem. Phys. 13, 2356–2366 (2010)
G. Jeschke, V. Chechik, P. Ionita, A. Godt, H. Zimmermann, J. Banham, C.R. Timmel, D. Hilger, H. Jung, Appl. Magn. Reson. 30, 473–498 (2006)
Acknowledgments
This work has been supported by the CARIPARO Foundation (M3PC project) by the MIUR (PRIN2010-2011 prot. 2010FM38P_004). A special acknowledgement is due to Giovanni Giacometti to whom this issue is dedicated, for stimulating with his personal interest and involvement, the research of the authors in the field of photosynthesis and bio-hydrogen production.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Maso, L., Galazzo, L., Vallese, F. et al. A conformational study of the GTPase domain of [FeFe]-hydrogenase maturation protein HydF by PELDOR spectroscopy. Appl Magn Reson 46, 465–479 (2015). https://doi.org/10.1007/s00723-015-0641-z
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00723-015-0641-z