Abstract
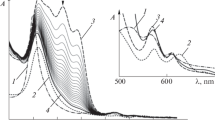
Ultraviolet and visible spectroscopy was applied to characterize and to measure the concentration of β-carotene dissolved in a dioxane and water mixture. The reaction of β-carotene in the presence of nitrite anion and acid medium was studied at different temperatures. The reaction systems were homogeneous and were kept anaerobic. Pseudo-first-order rate constants in respect of β-carotene were measured in the range from 293 to 313 K and pH 5.8 ± 0.2. The energy of activation was calculated to be E a = 67.2 ± 3.4 kJ/mol. We interpolate a value that may have biological interest, k β-carotene(310 K) = (9.70±0.78) · 10−3 s−1, in the presence of 9.3 · 10−3 M nitrite anion. Electron paramagnetic resonance spectroscopy was applied to characterize and quantify a persistent intermediate radical generated in the reaction system described. The recorded spectra showed triplet-type signals with a peak-to-peak value of 12.7 G. Nearly the same triplet radical-type intermediates were detected when studying the following reaction systems in pure dioxane: nitrogen dioxide (NO2)/β-carotene, nitric oxide (NO)/β-carotene and NO/NO2/β-carotene. Therefore, we proposed that the nitrogen oxides have also been intermediates in the reaction system of β-carotene, nitrite anion and acid medium, in the dioxane and water mixture. A mechanism was proposed and checked by employing the chemical kinetics simulation. The explanations developed would lead to a better understanding of the behavior of carotenoids in the presence of nitrite anion and nitrogen oxides.
Similar content being viewed by others
References
Halliwell, B., Gutteridge, J.M.C.: Free Radicals in Biology and Medicine, 3rd edn., pp. 220–225. Oxford University Press, New York (2001)
Mayo, F.R.: Acc. Chem. Res. 1, 193–201 (1968)
Burton, G.W., Ingold, K.U.: Science 224, 569–573 (1984)
Kennedy, T.A., Liebler, D.C.: Chem. Res. Toxicol. 4, 290–295 (1991)
Liebler, D.C., McClure, T.D.: Chem. Res. Toxicol. 9, 8–11 (1996)
Yamauchi, R., Miyake, N., Inoue, H., Kato, K.: J. Agric. Food Chem. 41, 708–713 (1993)
Mortensen, A., Skibsted, L.H., Sampson, J., Rice-Evans, C., Everett, S.A.: FEBS Lett. 418, 91–97 (1997)
Everett, S.A., Dennis, M.F., Patel, K.B., Maddix, S., Kundu, S.C., Wilson, R.L.: J. Biol. Chem. 271, 3988–3994 (1996)
Mortensen, A., Skibsted, L.H.: FEBS Lett. 426, 392–396 (1998)
Samokyszyn, V.M., Marnett, L.J.: J. Biol. Chem. 262, 14119–14133 (1987)
Pryor, W.A., Lightsey, J.W.: Science 214, 435–437 (1981)
Goldstein, S., Merenyi, G., Samuni, A.: J. Am. Chem. Soc. 126, 15694–15701 (2004)
Ignarro, L.J., Fukuto, J.M., Griscavage, J.M., Rogers, N.E., Byrns, R.E.: Proc. Natl. Acad. Sci. USA 90, 8103–8107 (1993)
Olson, O.E., Nelson, D.L., Emerick, R.: J. Agric. Food Chem. 11, 140–143 (1963)
Mendiara, S.N., Sagedahl, A., Perissinotti, L.J.: Appl. Magn. Reson. 20, 275–287 (2001)
Bianchi, E.M., Giesser, R., Sigel, H.: Helv. Chim. Acta 88, 406–425 (2005)
George, R.E., Woolley, E.M.: J. Solution Chem. 1, 279–289 (1972)
Mendiara, S.N., Perissinotti, L.J.: Appl. Magn. Reson. 25, 323–346 (2003)
Pérez-Gálvez, A., Mínguez-Mosquera, M.I.: J. Agric. Food Chem. 52, 632–637 (2004)
Mortensen, A., Skibsted, L.H.: J. Agric. Food Chem. 48, 279–286 (2000)
Chen, B.H., Peng, H.Y., Chen, H.E.: J. Agric. Food Chem. 43, 1912–1918 (1995)
Mallik, B., Jain, K.M., Misra, T.N.: Biochem. J. 189, 547–552 (1980)
Gabr, I., Patel, R.P., Symons, M.C.R., Wilson, M.T.: J. Chem. Soc. Chem. Commun. 1995, 915–916 (1995)
Lide, D.R. (ed.): CRC Handbook of Chemistry and Physics, 84th edn. CRC Press, Boca Raton, Fla. (2003)
Greenwood, N.N., Earnshaw, A.: Chemistry of the Elements, pp. 521–523. Butterworth-Heinemann, Oxford (1997)
Napolitano, A., Panzella, L., Savarese, M., Sacchi, R., Giudicianni, I., Paolillo, L., d’Ischia, M.: Chem. Res. Toxicol. 17, 1329–1337 (2004)
Park, J.Y., Lee, Y.N.: J. Phys. Chem. 92, 6294–6302 (1988)
Yamasaki, H.: Philos. Trans. R. Soc. Lond. B 355, 1477–1488 (2000)
Butler, A.R., Ridd, J.H.: Nitric Oxide 10, 20–24 (2004)
Hill, T.J., Land, E.J., McGarvey, D.J., Schalch, W., Tinkler, J.H., Truscott, T.G.: J. Am. Chem. Soc. 117, 8322–8326 (1995)
Mortensen, A., Skibsted, L.H.: Free Radic. Res. 25, 355–368 (1996)
Pryor, W.A., Lightsey, J.W., Church, D.F.: J. Am. Chem. Soc. 104, 6685–6692 (1982)
Kikugawa, K., Hiramoto, K., Tomiyama, S., Asano, Y.: FEBS Lett. 404, 175–178 (1997)
Park, J.S.B., Walton, J.C.: J. Chem. Soc. Perkin Trans. 2 1997, 2579–2583 (1997)
Moore, J.W., Pearson, R.G.: Kinetics and Mechanism, 3rd edn., p. 240. Wiley, New York (1981)
Geddes, J.A.: J. Am. Chem. Soc. 55, 4832–4837 (1933)
Åkerlöf, G., Short, O.A.: J. Am. Chem. Soc. 58, 1241–1243 (1936)
Hovorka, F., Schaefer, R.A., Dreisbach, D.: J. Am. Chem. Soc. 58, 2264–2267 (1936)
Geoffroy, M., Lambelet, P., Richert, P.: J. Agric. Food Chem. 48, 974–978 (2000)
Nicolescu, A.C., Zavorin, S.I., Turro, N.J., Reynolds, J.N., Thatcher, G.R.J.: Chem. Res. Toxicol. 15, 985–998 (2002)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mendiara, S.N., Baquero, R.P., Katunar, M.R. et al. Reaction of β-carotene with nitrite anion in a homogeneous acid system. An electron paramagnetic resonance and ultraviolet-visible study. Appl Magn Reson 35, 549–567 (2009). https://doi.org/10.1007/s00723-009-0185-1
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00723-009-0185-1