Abstract
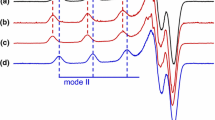
α-Synuclein (αS) is a small natively unfolded protein whose interactions with Cu2+ have been proposed to play a role in Parkinson’s disease (PD). We recently studied the Cu2+ coordination of recombinant human αS using electron paramagnetic resonance spectroscopy and identified two coordination modes at physiological pH, one anchored upon the amino terminus (mode 1) and the other anchored upon the side chain of His50 (mode 2). Here we report the Cu2+ coordination of the A30P, E46K and A53T mutants associated with inherited forms of PD. At physiological pH, the same two Cu2+ coordination modes were adopted by each of the familial mutants. The spectrum of Cu2+/αS(A53T) was very similar to the spectrum of the native Cu2+/αS complex; however, mode 2 coordination was marginally higher in the spectrum of Cu2+/αS(E46K) and considerably more favored in the Cu2+/αS(A30P) complex. The alteration in only the relative proportion of modes 1 and 2 suggests the familial mutations introduce structural changes of the protein backbone that indirectly affect the stability, but not the identity, of the native Cu2+ coordination modes.

Similar content being viewed by others
Notes
M29-D30-56 = NH2-M29-D30-GKTKEGVLYV40GSKTKEGVVH50GVATVA56-NH2). M26-D27-56(A30P,A53T) = NH2-M26-D27-EAPGKTKEGVLYV40GSKTKEGVVH50GVTTVA56-NH2). The role of Met1 and Asp2 residues in the full-length protein was modeled by appending a Met-Asp sequence to the N-terminus of the model complexes
References
R.E. Burke, Neurologist 10, 75–81 (2004)
J.C. Rochet, T.F. Outeiro, K.A. Conway, T.T. Ding, M.J. Volles, H.A. Lashuel, R.M. Bieganski, S.L. Lindquist, P.T. Lansbury, J. Mol. Neurosci. 23, 23–34 (2004)
R. Krüger, W. Kuhn, T. Müller, D. Woitalla, M. Graeber, S. Kösel, H. Przuntek, J.T. Epplen, L. Schöls, O. Riess, Nat. Genet. 18, 106–108 (1998)
M.H. Polymeropoulos, C. Lavedan, E. Leroy, S.E. Ide, A. Dehejia, A. Dutra, B. Pike, H. Root, J. Rubenstein, R. Boyer, E.S. Stenroos, S. Chandrasekharappa, A. Athanassiadou, T. Papapetropoulos, W.G. Johnson, A.M. Lazzarini, R.C. Duvoisin, G. Di Iorio, L.I. Golbe, R.L. Nussbaum, Science 276, 2045–2047 (1997)
J.J. Zarranz, J. Alegre, J.C. Gómez-Esteban, E. Lezcano, R. Ros, I. Ampuero, L. Vidal, J. Hoenicka, O. Rodriguez, B. Atarés, V. Llorens, E.G. Tortosa, T. del Ser, D.G. Muñoz, J.G. de Yebenes, Ann. Neurol. 55, 164–173 (2004)
R. Borghia, R. Marcheseb, A. Negroc, L. Marinellia, G. Forlonid, D. Zaccheoa, G. Abbruzzeseb, M. Tabatona, Neurosci. Lett. 287, 65–67 (2000)
H.-J. Lee, S. Patel, S.-J. Lee, J. Neurosci. 25, 6016–6024 (2005)
O.M. El-Agnaf, S.A. Salem, K.E. Paleologou, L.J. Cooper, N.J. Fullwood, M.J. Gibson, M.D. Curran, J.A. Court, D.M.A. Mann, S. Ikeda, M.R. Cookson, S. Hardy, D. Allsop, FASEB J. 17, 1945–1947 (2003)
Q.-X. Li, S.S. Mok, K.M. Laughton, C.A. McLean, R. Cappai, C.L. Masters, J.G. Culvenor, M.K. Horne, Exp. Neurol. 204, 583–588 (2007)
R.M. Rasia, C.W. Bertoncini, D. Marsh, W. Hoyer, D. Cherny, M. Zweckstetter, C. Griesinger, T.M. Jovin, C.O. Fernández, Proc. Natl. Acad. Sci. 102, 4294–4299 (2005)
O.M.A. El-Agnaf, R. Jakes, M.D. Curran, D. Middleton, R. Ingenito, E. Bianchi, A. Pessi, D. Neille, A. Wallace, FEBS Lett. 440, 71–75 (1998)
S. Turnbull, B.J. Tabner, O.M.A. El-Agnaf, S. Moore, Y. Davies, D. Allsop, Free Rad. Biol. Med. 30, 1163–1170 (2002)
B.J. Tabner, S. Turnbull, O.M.A. El-Agnaf, D. Allsop, Free Rad. Biol. Med. 32, 1076–1083 (2002)
T. Kowalik-Jankowska, A. Rajewska, E. Jankowska, K. Wiśniewska, Z. Grzonka, J. Inorg. Biochem. 100, 1623–1631 (2006)
T. Kowalik-Jankowska, A. Rajewska, E. Jankowska, Z. Grzonka, Dalton Trans. 4197–4206 (2007)
D. Kaur, F. Yantiri, S. Rajagopalan, J. Kumar, J.Q. Mo, R. Boonplueang, V. Viswanath, R. Jacobs, L. Yang, M.F. Beal, D. DiMonte, I. Velitaskis, L. Ellerby, R.A. Cherny, A.I. Bush, J.K. Andersen, Neuron 37, 899–909 (2003)
S.C. Drew, S.L. Leong, C.L.L. Pham, D.J. Tew, C.L. Masters, L.A. Miles, R. Cappai, K.J. Barnham, K.J. Barnham, J. Am. Chem. Soc. 130, 7766–7773 (2008)
R. Cappai, S.L. Leck, D.J. Tew, N.A. Williamson, D.P. Smith, D. Galatis, R.A. Sharples, C.C. Curtain, F.E. Ali, R.A. Cherny, J.G. Culvenor, S.P. Bottomley, C.L. Masters, K.J. Barnham, A.F. Hill, FASEB J. 19, 1377–1379 (2005)
T. Kowalik-Jankowska, A. Rajewska, K. Wiśniewska, Z. Grzonka, J. Jezierska, J. Inorg. Biochem. 99, 2282–2291 (2005)
T. Kowalik-Jankowska, A. Rajewska, E. Jankowska, Z. Grzonka, Dalton Trans. 5068–5076 (2006)
M. Bataille, G. Formicka-Kozlowska, H. Kozlowski, L.D. Pettit, I. Steel, J. Chem. Soc. Chem. Commun. 231–232 (1984)
Acknowledgments
This work was supported in part by a Program Grant administered by the National Health and Medical Research Council (NHMRC) of Australia. K.J. Barnham is a NHMRC Senior Research Fellow. We thank Denise Cappai for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Drew, S.C., Tew, D.J., Masters, C.L. et al. Copper Coordination by Familial Mutants of Parkinson’s Disease-Associated α-Synuclein. Appl Magn Reson 36, 223–229 (2009). https://doi.org/10.1007/s00723-009-0020-8
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00723-009-0020-8