Summary
Background
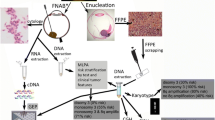
Genetic analysis of choroidal melanoma is frequently used to estimate the risk of metastatic spread of the tumor. Obtaining a biopsy for genetic analysis, however, can be difficult and sometimes unsuccessful. We evaluated the feasibility and accuracy of genetic testing using array comparative genomic hybridization (CGH) after radiotherapy, from tumor samples obtained by endoresection or after secondary enucleation.
Material and methods
Fifteen choroidal melanoma samples obtained after radiotherapy (Ruthenium-106 plaque brachytherapy or Gamma-Knife radiosurgery) were analyzed by array CGH to detect chromosomal aberrations (monosomy 3 and trisomy 8q), and the results were compared with pre-irradiation findings in five cases.
Results
Array CGH was successfully performed in all 15 cases. Time from radiotherapy to obtaining the sample for cytogenetic testing was between 14 and 879 days. Results of post-radiotherapy genetic analysis did not differ from pre-radiotherapy findings.
Conclusion
Post-radiation CGH appears to be a promising option for prognostic testing if a first biopsy before radiotherapy failed or was not performed. It could be useful to avoid an additional surgical procedure before radiotherapy if vitrectomy or endoresection is planned after radiotherapy.
Zusammenfassung
Hintergrund
Die genetische Untersuchung von Aderhautmelanomen ist eine zunehmend häufiger eingesetzte Methode, um das Risiko der Metastasenentwicklung bei PatientInnen mit Aderhautmelanomen einzuschätzen. Die Gewinnung einer Gewebeprobe zur Durchführung der Untersuchung ist jedoch manchmal schwierig und nicht in allen Fällen erfolgreich. Wir untersuchten die Durchführbarkeit und Genauigkeit der genetischen Untersuchung von Aderhautmelanomen mittels array comparative genomic hybridization (CGH) nach Strahlentherapie, an mittels Endoresektion oder nach Enukleation gewonnenem Tumormaterial.
Material und Methode
Fünfzehn Gewebeproben von strahlentherapeutisch behandelten Aderhautmelanomen wurden mittels array-CGH auf Veränderungen an den Chromosomen 3 und 8 untersucht (Monosomie 3, Trisomie 8q). Die Ergebnisse wurden mit den in fünf Fällen vorliegenden Resultaten der genetischen Untersuchung vor Bestrahlung verglichen.
Resultate
Die array CGH konnte in allen 15 Fällen nach Bestrahlung erfolgreich durchgeführt werden. Die Zeitspanne von der Bestrahlung bis zur genetischen Untersuchung lag zwischen 14 und 879 Tagen. Die Resultate der genetischen Untersuchung nach Bestrahlung unterschieden sich nicht von den Ergebnissen der in 5 Fällen vorliegenden Ergebnissen vor der Bestrahlung.
Schlußfolgerung
Die array CGH von Aderhautmelanomen nach Strahlentherapie erscheint eine vielversprechende Option zur prognostischen Unteruchung in den Fällen zu sein, in denen eine Biopsie vor Bestrahlung nicht durchgeführt wurde oder nicht erfolgreich war. Im Falle einer geplanten Endoresektion nach Bestrahlung, könnte auf einen zusätzlichen Eingiff zu Biopsie vor der Bestrahlung verzichtet werden.

Similar content being viewed by others
References
Singh AD, Topham A. Incidence of uveal melanoma in the United States: 1973–1997. Ophthalmology. 2003;110:956–61.
Chang AE, Karnell LH, Menck HR. The National Cancer Data Base report on cutaneous and noncutaneous melanoma. a summary of 84,836 cases from the past decade. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer. 1998;83:1664–78.
Singh AD, Turell ME, Topham AK. Uveal melanoma: trends in incidence, treatment, and survival. Ophthalmology. 2011;118:1881–5.
Singh AD, Topham A. Survival rates with uveal melanoma in the United States: 1973–1997. Ophthalmology. 2003;110:962–5.
Mudhar HS, Parsons MA, Sisley K, et al. A critical appraisal of the prognostic and predictive factors for uveal malignant melanoma. Histopathology. 2004;45:1–12.
Prescher G, Bornfeld N, Becher R. Nonrandom chromosomal abnormalities in primary uveal melanoma. J Natl Cancer Inst. 1990;82:1765–9.
Sisley K, Rennie IG, Cottam DW, et al. Cytogenetic findings in six posterior uveal melanomas: involvement of chromosomes 3, 6, and 8. Genes, chromosomes & cancer. 1990;2:205–9.
Prescher G, Bornfeld N, Hirche H, et al. Prognostic implications of monosomy 3 in uveal melanoma. Lancet. 1996;347:1222–5.
Sisley K, Rennie IG, Parsons MA, et al. Abnormalities of chromosomes 3 and 8 in posterior uveal melanoma correlate with prognosis. Genes, chromosomes & cancer. 1997;19:22–8.
Damato B, Coupland SE. Translating uveal melanoma cytogenetics into clinical care. Arch Ophthalmol. 2009;127:423–9.
Damato B, Duke C, Coupland SE, et al. Cytogenetics of uveal melanoma: a 7-year clinical experience. Ophthalmology. 2007;114:1925–31.
Midena E, Bonaldi L, Parrozzani R et al In vivo detection of monosomy 3 in eyes with medium-sized uveal melanoma using transscleral fine needle aspiration biopsy. European journal of ophthalmology. 2006;16:422–5.
Young TA, Burgess BL, Rao NP, et al. Transscleral fine-needle aspiration biopsy of macular choroidal melanoma. Am J Ophthalmol. 2008;145:297–302.
Wackernagel W, Schmutzer M, Mayer CF, et al. Biopsy of intraocular tumors in clinically uncertain diagnosis. Spektrum der Augenheilkunde. 2005;19:171–5.
Bechrakis NE, Foerster MH. Neoadjuvant proton beam radiotherapy combined with subsequent endoresection of choroidal melanomas. Int Ophthalmol Clin. 2006;46:95–107.
Singh AD, Triozzi PL. Endoresection for choroidal melanoma: palliative or curative intent? Br J Ophthalmol. 2008;92:1015–6.
Bechrakis NE, Blatsios G, Schmid E, et al. Surgical resection techniques of large uveal melanomas. Spektrum Der Augenheilkunde. 2010;24:17–22.
Herwig M, Eter N. 23-gauge versus 20-gauge vitrectomy: analysis of 110 consecutive cases undergoing epiretinal membrane peeling and macular hole repair. Spektrum Der Augenheilkunde. 2012;26:172–4.
Bezatis A, Laufenbock C, Zehetner C, Kieselbach G, Kralinger M, et al. Macular hole surgery: anatomical and functional results. Spektrum Der Augenheilkunde. 2011;25:302–5.
Tarmann L, Wedrich A, Hass A, et al. Limited vitrectomy with intravitreal bevacizumab, rt-PA and gas for submacular hemorrhage due to age-related macular degeneration. Spektrum Der Augenheilkunde. 2012;26:197–201.
Mayer CF, Langmann G, Wackernagel W, et al. Globe preservation and visual function after endoresection and Gamma-Knife radiosurgery for uveal melanomas. Spektrum der Augenheilkunde. 2009;23:347–52.
Shields CL, Ganguly A, Materin MA, et al. Chromosome 3 analysis of uveal melanoma using fine-needle aspiration biopsy at the time of plaque radiotherapy in 140 consecutive cases. Transactions of the American Ophthalmological Society. 2007;105:43–52; discussion–3.
Young TA, Rao NP, Glasgow BJ, et al. Fluorescent in situ hybridization for monosomy 3 via 30-gauge fine-needle aspiration biopsy of choroidal melanoma in vivo. Ophthalmology. 2007;114:142–6.
Foster WJ, Harbour JW, Holekamp NM, et al. Pars plana vitrectomy in eyes containing a treated posterior uveal melanoma. Am J Ophthalmol. 2003;136:471–6.
Glasgow BJ, Brown HH, Zargoza AM, et al. Quantitation of tumor seeding from fine needle aspiration of ocular melanomas. Am J Ophthalmol. 1988;105:538–46.
Suesskind D, Ulmer A, Schiebel U, et al. Circulating melanoma cells in peripheral blood of patients with uveal melanoma before and after different therapies and association with prognostic parameters: a pilot study. Acta ophthalmologica. 2011;89:17–24.
Garcia-Arumi J, Zapata MA, Balaguer O, et al. Endoresection in high posterior choroidal melanomas: long-term outcome. Br J Ophthalmol. 2008;92:1040–5.
Schefler AC, Gologorsky D, Marr BP et al Extraocular extension of uveal melanoma after fine-needle aspiration, vitrectomy, and open biopsy. JAMA ophthalmology. 2013;131:1220–4.
Pe’er J, Stefani FH, Seregard S, et al. Cell proliferation activity in posterior uveal melanoma after Ru-106 brachytherapy: an EORTC ocular oncology group study. Br J Ophthalmol. 2001;85:1208–12.
Tschentscher F, Husing J, Holter T et al Tumor classification based on gene expression profiling shows that uveal melanomas with and without monosomy 3 represent two distinct entities. Cancer research. 2003;63:2578–84.
Onken MD, Worley LA, Ehlers JP et al Gene expression profiling in uveal melanoma reveals two molecular classes and predicts metastatic death. Cancer research. 2004;64:7205–9.
Acknowledgments
We thank Anna Obenauf, PhD, from Department of Human Genetics, Medical University Graz, for the support in genetic analysis of the samples.
Conflict of interest
Werner Wackernagel, Lisa Tarmann, Christoph Mayer, Gerald Langmann, and Andreas Wedrich declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Data were presented in part at the 51st meeting of the Austrian Ophthalmological Society, ARVO Science Day, May 14th, 2010, Zell am See, Austria.
Rights and permissions
About this article
Cite this article
Wackernagel, W., Tarmann, L., Mayer, C. et al. Genetic analysis of uveal melanoma by array comparative genomic hybridization before and after radiotherapy. Spektrum Augenheilkd. 27, 286–291 (2013). https://doi.org/10.1007/s00717-013-0195-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00717-013-0195-0