Abstract
Determination of highly siderophile element (HSE; Au, Pt, Pd, Ir, Os, Rh and Ru) concentrations in relatively unweathered and unaltered marine shales from the Barberton, Witwatersrand and Transvaal supergroups in the Kaapvaal Craton revealed systematic differences, interpreted to reflect secular changes in the HSE content of Mesoarchaean to Palaeoproterozoic seawater. Most of the studied marine shales have HSE concentrations in the range given for average Archaean crustal rocks (0.5–5 ppb), with the exception of the shales in the Witwatersrand Supergroup. These shales contain up to three times more HSE, independent of source rock lithology in the granitoid-greenstone-dominated hinterland. Although sedimentary pyrite incorporated gold from synsedimentary to early diagenetic waters, its modal proportion (<3 vol.%) is too small to account for the total amount of Au and PGE in the studied marine shales. Instead, our results suggest that in addition to contributions from pyrite, some colloidal gold was attached to clay-sized sediments during source area weathering. Probably, colloidal gold and some of the platinum group elements were mechanically aggregated during sediment suspension and deposited synchronously with the host marine sediments.
Similar content being viewed by others
Introduction
Gold and platinum group elements (PGE, i.e. Pt, Pd, Ir, Os, Rh and Ru) comprise a select group of transition metals that have highly siderophile affinities and are, therefore, mainly concentrated in the Earth’s core. Rudnick and Gao (2005) estimated average HSE concentrations in the upper continental crust to be 1.50 ppb Au, 0.50 ppb Pt and 0.52 ppb Pd. The average Au and PGE content in modern seawater are extremely low, with <0.01 to 0.06 ppt Au, 0.04 to 0.07 ppt Pt, and 0.04 ppt Pd (Goldberg 1987; Falkner and Edmond 1990; Koide et al. 1991; Mashio et al. 2017).
In open oceans, gold and PGEs are present as a mixture of dissolved complexes, colloids, nanoparticles, aqueous clusters, and organic complexes which may be adsorbed onto clays (Seward 1989). Highly siderophile elements have an affinity for sulphides and carbonaceous matter (Lorand et al. 2008; Southam et al. 2009). Thus, carbonaceous sulphidic shales are typically enriched in HSE (Seward 1989; Lehmann et al. 2007). In general, low-oxygen bottom waters in the depositional environment are inferred from metal-rich black shales. In addition, a recent study by Johnson et al. (2017) revealed that elevated oxygen in the atmosphere and low oxygen bottom waters are a common feature of the Phanerozoic and gave rise to metal-enriched organic carbon-rich shales. To obtain information on possible secular variations in HSE concentrations in Archaean to Palaeoproterozoic sea water, we studied marine shales with low sulphide and organic carbon contents.
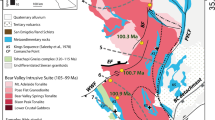
Published gold values in settings other than exploited auriferous conglomerates (reefs) are rare in the literature on the Kaapvaal Craton, mainly because interest is virtually restricted to economically mineable rocks such as the Witwatersrand conglomerates and carbon seams. Previous data on Archaean shales suggest a generally low abundance of HSE but with considerable variation across stratigraphic levels (Meyer and Saager 1985). These variations form the focus of the present study. Do these variations reflect differences in sediment provenance and/or differences in the coeval seawater chemistry? Could they be due to variations in the HSE flux into the ocean and/or an effective trapping mechanism? In an effort to contribute to the understanding of Au and PGE distribution and to specifically address the mechanisms responsible for concentrating these metals, we sampled marine shales of the laterally extensive cratonic sedimentary cover successions of the Kaapvaal Craton in South Africa (Fig. 1).
Surface and subsurface distribution of the main Archaean stratigraphic units of the Kaapvaal Craton, showing the known extent of the Witwatersrand Basin, the fill of which comprises the West Rand and Central Rand groups; blue line delineates trace of the Black Reef Formation at the base of Neoarchaean to early Palaeoproterozoic Transvaal Basin; from Frimmel and Hennigh (2015); stars indicate positions of samples (in drill core or underground). Fm. = Formation (modified after Frimmel 2014). Ages: Barberton Supergroup and Dominion Group (Lowe and Byerly 1999; Poujol et al. 2003; Schoene et al. 2009; Zeh et al. 2009); Witwatersrand Supergroup (Kositcin and Krapež 2004); Ventersdorp and Transvaal Supergroup (Gumsley et al. 2018)
Geochemistry of marine shales in the Kaapvaal Craton
A large amount of geochemical data has been obtained from numerous (meta-)sedimentary rock types throughout the Kaapvaal Craton, mainly mineralised conglomerate, quartzite, and banded iron formation (BIF). Many of these rock types have been analysed to constrain source rock lithology, topographic relief, source area weathering patterns, tectonic setting, sediment transport history, and diagenetic modifications (e.g. Wronkiewicz and Condie 1987; Toulkeridis et al. 2015; Nwaila et al. 2017). Geochemical studies of shales (some of which experienced low-grade metamorphism) in the Kaapvaal Craton are limited in number (Guy 2012). Shale units of the Barberton Supergroup have been, by comparison, more intensely studied and used to constrain provenance, source area weathering, and post-depositional events (e.g. Taylor and McLennan 1985; Hofmann 2005; Toulkeridis et al. 2015). The HSE content of the Barberton Supergroup shales has remained, however, largely untested.
The Witwatersrand Supergroup shales have been studied to assess sediment provenance (e.g. Fuller et al. 1981; Wronkiewicz and Condie 1987), characterise post-depositional alteration (e.g. Phillips and Powell 2015; Fuchs et al. 2016a), and to constrain Archaean palaeoenvironmental conditions (e.g. Watanabe et al. 1997; Guy et al. 2010; Smith et al. 2013). More recently, Nwaila et al. (2017) used shale geochemical data to deduce a high rate of chemical weathering in the hinterland of the Witwatersrand Basin as well as on intrabasinal erosion surfaces of the Witwatersrand strata.
The sedimentary succession of the overlying Transvaal Supergroup records a period of extreme palaeoenvironmental changes on Earth, including global ice ages, significant global warming, and the rise of atmospheric oxygen (Hannah et al. 2004). Genetic stratigraphic models have been developed for the Transvaal Supergroup (e.g. Coetzee et al. 2006) through the integration of sequence stratigraphy, sedimentology, and geochemical analysis of shales and other sedimentary rocks. Published HSE data on marine shales are, however, limited (Meyer and Robb 1996) and most of them refer to altered shales of the Chuniespoort Group in which gold is probably due to post-depositional secondary enrichment.
Various studies on the origin of Au and PGE in greenstone belts and sedimentary successions have focused on syngenetic models (e.g. Ridler 1970; Hutchinson et al. 1971; Large et al. 2013), igneous intrusions (e.g. Xue et al. 2013), and the mantle (e.g. Hronsky et al. 2012) as the three most likely sources of these metals. More recently, seawater has been considered the ultimate source of dissolved gold that becomes concentrated in shales through the growth of syngenetic and diagenetic pyrite (e.g. Large et al. 2011, 2015; Tomkins 2013). This model suggests that the gold is sourced from seawater, either as dissolved complexes, or adsorbed on particulate organic matter, Fe- and Mn-hydroxides, and clays. A more comprehensive geochemical dataset covering marine shales of the Kaapvaal Craton may help to constrain changes in the mode of Au transfer into the oceans and marine sediments therein. This information could assist in any discussion of the origin of the various detrital components, including gold and detrital PGE in the sediments. Comparable studies on younger sequences have been, for example, those on provenance and formation mechanisms of gold deposits in New Zealand’s Otago schist belt, the state of Victoria in Australia, and Scotland’s Dalradian metasedimentary belt (Pitcairn et al. 2006; Pitcairn 2010). In this paper, we follow a geochemical approach to illustrate variations in the HSE content of Archaean and Palaeoproterozoic marine shales and suggest a conceptual model that might explain HSE concentration in these sediments.
Geological background, regional metamorphism and alteration
This study focuses on three prominent mega-sequences of the Kaapvaal Craton (Fig. 1), namely the Barberton, Witwatersrand, and Transvaal supergroups. The Barberton Supergroup is one of the world’s best-preserved Palaeo- to Mesoarchaean volcano-sedimentary successions and is exposed in the Barberton Greenstone Belt near the southeastern edge of the Kaapvaal Craton (Armstrong et al. 1990). The Barberton Greenstone Belt consists of a northern and a southern terrane, separated by the northeast-trending Saddleback-Inyoka fault system (Heubeck and Lowe 1994). The Barberton Supergroup comprises the Onverwacht Group (ca. 3.55–3.30 Ga, 15.2 km-thick, Poujol et al. 2003), which consists of submarine ultramafic-mafic volcanic rocks and minor felsic volcanic and silicified sedimentary rocks. The Fig Tree Group (ca. 3.26–3.23 Ga, 1.5–2 km thick, Byerly et al. 1996) lies above the Onverwacht Group and consists of marine shale, greywacke, and felsic volcaniclastic rocks with minor conglomerates. The Moodies Group (ca. 3.224–3.210 Ga, 3.5 km-thick, Heubeck et al. 2013) was deposited above the Fig Tree Group and it is a succession of fluvial sandstone and conglomerate with minor shallow marine shale and banded iron formation (Heubeck 2014).
The Witwatersrand Supergroup, located in the central portion of the Kaapvaal Craton (Fig. 1), contains one of the best-preserved records of marine and fluvial sedimentation on an Archaean craton (McCarthy 2006). The Witwatersrand Supergroup has been subdivided into the lower 2.99–2.91 Ga West Rand Group and the upper 2.90–2.79 Ga Central Rand Group with the latter being well-known for hosting the world’s largest concentration of gold and huge amounts of uranium (for reviews on this ore province see Robb and Meyer 1995; Phillips and Law 2000; Frimmel et al. 2005; McCarthy 2006; Frimmel 2014; Tucker et al. 2016). It rests on the 2.7 km-thick, and locally uraniferous, volcano-siliciclastic 3.07 Ga Dominion Group rocks in the central part of the craton, which in turn overlie a Palaeo- to Mesoarchaean granitoid–greenstone basement. In the current study, two regionally persistent marine shale units were sampled in the Witwatersrand Supergroup, namely the Roodepoort Formation (Jeppestown Subgroup), which is, stratigraphically, the highest major marine shale unit in the West Rand Group, and the Booysens Formation (Johannesburg Subgroup), which is the only marine shale unit within the Central Rand Group. Detailed geological descriptions of the Roodepoort and Booysens formations are given in Nwaila et al. (2017). At the top of the Witwatersrand Supergroup, separated by a major regional angular unconformity, are predominantly metavolcanic rocks of the 2.79 Ga (Gumsley et al. 2018) >3.5 km-thick Ventersdorp Supergroup.
Following deposition of the Ventersdorp Supergroup, the Kaapvaal Craton was subjected to large-scale erosion resulting in a major unconformity at the contact with the overlying Neoarchaean to Palaeoproterozoic Transvaal Supergroup rocks. The Transvaal Supergroup is structurally preserved in the Transvaal and Griqualand West sub-basins and covers an area of at least 500,000 km2. The Vryburg Formation, located at the base of the Transvaal Supergroup in the Griqualand West sub-basin, has been used to establish the maximum age limit for the supergroup at 2.67 Ga (U-Pb zircon ages; Gutzmer and Beukes 1998) and 2.66 Ga (Sumner and Beukes 2006). The Black Reef Formation, one of the subjects of this study, is regarded as its stratigraphic equivalent in the Transvaal sub-basin (Eriksson et al. 2006). The minimum age of Transvaal Supergroup deposition is given by U-Pb zircon ages of 2.056 Ga from the Rustenburg Layered Suite of the Bushveld Igneous Complex (Zeh et al. 2015) and 2.057 Ga for the Kwaggasnek Formation of the Rooiberg Group (Harmer and Armstrong 2000). Locally, the Black Reef Formation (ca. 80 m-thick) contains a basal auriferous conglomerate, which grades upwards via quartzite into shallow marine black shale. The Black Reef Formation is conformably overlain by dolomite of the Malmani Subgroup of the 2.64 to 2.43 Ga Chuniespoort Group, and the formation’s upper part shows a transition into the basal beds of the Malmani Subgroup dolomite (Eriksson et al. 2006). The Chuniespoort Group is overlain by the ca. 2.35 to 2.25 Ga Pretoria Group (Eriksson et al. 2006). For this study, the shallow marine Black Reef Formation shales and shallow to deep marine shales of the Silverton Formation in the upper Pretoria Group (post-Great Oxidation Event) were sampled.
Even though there are no specific geothermometric data on metamorphic grade for some of the marine shales of the Kaapvaal Craton (e.g. Black Reef Formation), all the studied shales are likely to have experienced similar metamorphic conditions as those reported in the Barberton Greenstone Belt and Witwatersrand Supergroup. The Barberton Greenstone Belt underwent regional lower greenschist-facies metamorphism with peak metamorphic conditions of 350 ± 50 °C; muscovite and chlorite are widespread in the metamorphic mineral assemblage (Heubeck et al. 2016). Chloritoid occurs as a metamorphic mineral in some of the shales that are intercalated with quartzite (Weis and Wasserburg 1987). In the Witwatersrand Supergroup, a series of tectonic-metamorphic overprints have been documented. Robb et al. (1997), Frimmel and Minter (2002), Frimmel et al. (2005), and Dankert and Hein (2010) discussed these and outlined the major tectonic-metamorphic events: (i) ca. 2.83 Ga deformation near the western margin of the Witwatersrand Basin in response to accretion of an oceanic arc to the western margin of the Kaapvaal Craton; (ii) compression due to accretion of the Limpopo Belt followed by orogenic collapse, continental extension and deposition of the Ventersdorp Supergroup; (iii) thermal subsidence contemporaneous with the deposition of the 2.64 to 2.43 Ga Chuniespoort Group that led to low-grade burial metamorphism of the Witwatersrand strata; (iv) burial metamorphism of the Witwatersrand strata during the deposition of the ca. 2.35 to 2.25 Ga Pretoria Group after a major erosive phase; (v) inferred magmatic underplating and intrusion of large amounts of basic or ultrabasic melts into the lower to middle crust during the emplacement of the 2.056 Ga Bushveld Igneous Complex. This event led to a thermal metamorphic, and possibly metasomatic, overprint of the lower parts of the Witwatersrand basin fill; and (vi) the 2.023 Ga Vredefort meteorite impact (Kamo et al. 1996), which is thought to have created secondary permeability in the Witwatersrand strata. Later tectono-metamorphic overprints during orogenic events along the margins of the Kaapvaal Craton (i.e. the 2.0 to 1.8 Ga Kheis, the 1.2 to 1.0 Ga Namaqua, and the 0.60 to 0.50 Ga Pan-African orogenies) had little or no significant effect on the Witwatersrand basin fill. The above multistage tectonic-metamorphic overprints induced regional lower greenschist-facies conditions of 350 ± 50 °C at 1–3 kbar (Wallmach and Meyer 1990; Frimmel 1994; Phillips and Law 1994). Assessment of the extent of post-depositional alteration in marine shales of the Witwatersrand Supergroup revealed that such alteration, evident in the form of sericite and chlorite, was limited to local zones of hydrothermal fluid infiltration along fractures and contacts with coarser grained siliciclastic rocks (Nwaila et al. 2017).
Samples and lithogeochemical analyses
A total of 200 shale samples from the Barberton, Witwatersrand, and Transvaal supergroups (Fig. 1, Table 1 and Supplementary data S1) were analysed for major and trace elements, including siderophile element concentrations. The shales were sampled from drill cores and underground mining exposures to avoid recent weathering effects. The sampling strategy involved collecting marine shales that lack evidence of significant post-depositional alteration (i.e. quartz and calcite veins and secondary pyrite stringers), an aspect that was assessed with the help of sample profiles from zones of alteration into seemingly unaltered shale units. Samples were collected from laterally equivalent distal positions, thereby focusing on regionally persistent marine shales. The main sedimentary structures observed across all the sampled marine shales are lamination, horizontal stratification, soft sediment deformation, and shale drapes. Most, if not all, of these shales were deposited from generally low-energy flow with sedimentation dominated by suspension settling (Beukes 1995; Coward et al. 1995; Smith et al. 2013; Eriksson et al. 2006; Heubeck et al. 2016).
All samples were carefully washed with distilled water, dried, and examined visually. Samples free of veins or signs of mineralisation were crushed into coarse fragments (3 to 10 mm), using a jaw-crusher, and subsequently hand-picked to remove fragments that contained the original drill core surface. Picked fragments were further washed with distilled deionised water (several times to remove attached dust), dried, and powdered in an agate mill. Between each milling run, coarse quartz sand was processed to minimise cross-contamination between samples, and duplicate control samples were also processed. Major element concentrations were determined at the Institute of Geography and Geology, University of Würzburg, those of the trace elements at the GeoZentrum Nordbayern at the University of Erlangen-Nürnberg, and those of the HSE at Intertek Genalysis Laboratory in Perth, Australia,
Major and trace element analyses
Major and trace element concentrations (Supplementary data S1) were obtained by X-ray fluorescence (XRF) spectrometry and inductively coupled plasma mass spectrometry (ICP-MS) on fusion glass disks. Procedural blanks and several standard reference materials (AMIS0283, AMIS0192 and OREAS series (25a, 45d, 45e) standards) were used to monitor data quality. Loss on ignition was determined after heating previously dried powders to a temperature of 950 °CA. A PANalytical MINPAL4 XRF spectrometer was used to determine the major element concentrations. The instrument is equipped with a side-window Rh-tube with max 30 kV, max 1 mA, max 9 W, and an energy-dispersive Si drift detector. The sample chamber was flushed with He to lower the detection limits and reduce analytical errors. The analytical error for most of the elements are <0.10 % (relative), except for MgO (0.50 %) and Na2O (3.40 %). For more details, see http://www.geographie.uni-wuerzburg.de/geodynamik/analytische_ausstattung/.
An Agilent 7700× quadrupole ICP-MS equipped with a Photon-Machines Analyte 193 excimer laser ablation system and a Helex two-volume ablation cell was used to determine the trace element concentrations in lithium borate flux-fused beads. Helium was used as carrier gas. The analyses were performed at a laser repetition rate of 10 Hz, a laser pulse energy of 4 J·cm−2, and largest possible spot size (63 μm). Continuous line-scan analyses were conducted with a pre-ablation step and 5 μm/s scanning speed. Samples were assessed in cycles of approximately 15 analyses, and these were bracketed by analyses of each standard (AMIS0283, AMIS0192 and OREAS series (25a, 45d, 45e) standards). The GLITTER™ software package (van Achterbergh et al. 2001) was used for the offline selective integration of time-resolved signal intensities and the calculation of the element concentrations, detection limits, and 1σ uncertainties. The detection limits were 0.001 wt% for major elements, 0.50 ppm for trace elements (excluding Cr = 20 ppm, Sr = 0.20 ppm, Sc and V = 10 ppm, respectively, and Sn, W, Zn, and Zr = 1 ppm), and 0.10 ppm for REEs. The reported Fe2O3 content represents the total Fe content. All major and trace element data were normalised against the post-Archaean Australian shale (PAAS) composite values of Taylor and McLennan (1985) due to the absence of reliable Archaean reference data. Most of the recent literature also utilise the PAAS reference for comparative reasons, rather than an Archaean shale composite due to consistency of the former standard (Smith et al. 2013; Nwaila et al. 2017).
Lead collection fire assay, new pot with an ICP-MS finish (method FA25N/MS)
The Au and PGE concentrations were determined by fire assay following a dry procedure by furnace methods. Fire assaying by lead collection was used to pre-concentrate samples to determine the gold grade (Huffman et al. 1998). The Pb collection fire assay digestion is applicable to Au, Pt and Pd, but the other platinum group elements are lost, in varying amounts, during the cupellation stage. Lead fire assay is a more effective collection for Au than the nickel sulphide (NS25) collection (Intertek Genalysis Laboratory, Perth, Australia). Twenty-five grams of pulped sample were mixed with a PbO-based flux and ignited in a furnace. New pots were used to preclude the possibility of a minute amount of cross-contamination of the precious metals. The PbO was reduced to Pb by the presence of an organic reducing agent. The molten Pb metal was miscible with the precious metals but immiscible with the slag. The Pb thus collects the precious metals and sinks to the bottom of the melt where it coalesces to form a button. The melt was poured into a conical mould where it was allowed to cool. The Pb button was separated from the slag and hammered into a cube to facilitate handling. The cupellation stage involves oxidation of the Pb button. The Pb buttons were placed in magnesia cupels in a furnace where they melted and oxidised. The PbO was absorbed by the cupel, leaving the precious metals behind which coalesced into a prill due to surface tension. The presence of a small amount of silver in the flux allows for a prill of manageable size. This was transferred into a polypropylene test tube, digested in aqua regia, the volume measured, and diluted for analysis by ICP-MS. The use of sensitive instrumentation such as ICP-MS enables lower detection limits (e.g. Abou-Shakra 2013) such as 1 ppb for Au and 0.5 ppb for Pt and Pd. Internal standards were used to correct for instrument drift and plasma fluctuations. The results were corrected for the weight by the laboratory information management system. To provide the true analytical uncertainty on our data we use Au assay as an example. Gold content in the studied marine shales that are within or below detection limit (1 ppb) contribute 7 % of the total number of samples analysed (N = 45) for the Barberton Supergroup, 16 % of the total number of sampled analyses (N = 116) for the Witwatersrand group and 24 % of the total number of samples analysed (N = 30) for the Transvaal Supergroup assays. Based on duplicate measurements of the samples (Supplementary data S1: Table S9 Quality control certified reference materials, samples and detection limits), the %RSD is ≤10 % for all analyses. To test the accuracy and precision of the analyses, replicate analyses of each of the certified reference materials (AMIS0283 & AMIS0192 and OREAS series (25a, 45d, 45e) standards) were performed (Supplementary data S1: Table S9 Quality control certified reference materials, samples and detection limits).
Total organic carbon – total sulphur analysis
Total organic carbon (TOC) and total sulphur (TS) were analysed using an Eltra Infrared CS-2000 LECO analyser with a detection limit of 0.01 % at Intertek Laboratory in Perth, Australia. The pulped sample was weighed out and placed in a ceramic crucible. A fluxing agent was added to improve fluidity and oxidation of the carbon and sulphur. Heating was accomplished in a high-frequency induction furnace as this provides both speed and accuracy. Any sulphur or carbon was converted to SO2 or CO2, respectively. These gases absorb infrared radiation at specific wavelengths which are proportional to the concentration of the C or S in the sample. Any water in the sample was removed by passing the gases produced through magnesium perchlorate as water interferes with the analysis. Calibration was effected by a standard (OREAS 45d) of known C and S concentration and a control blank.
Statistical treatment of gold and platinum group element outliers
When the sampling design specifies a single measurement at each of n distinct locations, the nugget effect has a dual interpretation as either measurement error or spatial variation on a scale smaller than the smallest distance between any two points in the sample design, or any combination of these two effects (Goovaerts et al. 2016). These two components of the nugget effect can only be separately identified if the variance of the measurement error is either known or can be estimated directly using repeated measurements taken at coincident locations. In this study, we define outliers as extreme values that deviate from other observations on data, which may indicate variability in a measurement, experimental errors or observation that diverges from an overall HSE pattern on a sample. In general, outliers can also come in different categories such as point outliers, contextual outliers, or collective outliers. In this study, point outliers which are single data points that lay far from the rest of the distribution are the most dominant form (e.g. Fig 4c, drill core E1G). Prior to interpretation of the Au and PGE results, the Au and PGE assay data from the sample database were subjected to rigorous parametric distribution modelling and normality tests. The distribution of the collected sample points was examined by plotting the gold assay data as a histogram and conducting the non-parametric Anderson-Darling test for normality, which is one of three general normality tests (the other two being the Shapiro-Wilks and Skewness-Kurtosis All tests; Yazici and Yolacan 2005) designed to detect all departures from normality (i.e. the nugget effect). From these tests, it became apparent that the Au and PGE data display a skewed distribution. This is common for Au and PGE contents, where the largest population of the dataset falls under the lower limits and the upper limits are defined by limited number of samples (McLennan and Deutsch 2004). When the distribution of the continuous data is non-normal, transformations are applied to make the data as “normal” as possible to increase the validity of the associated statistical analyses (Krige 1960). Log-transformation is a widely used method to normalise skewed data in economic and mining geology, biomedical and psychosocial fields (McDonald 2014). The log transformation is arguably the most popular among the different types of transformations (e.g. normal scores, square-root and arcsine transformations) used to normalise skewed data.
In the case of our Au and PGE dataset, the original data follows a log-normal distribution, thus allowing the treatment of outliers through the use of the natural log (ln) transformations, which reduces the skewness and variability of the data. Since the log-transformation can only be used for positive outcomes, a small positive constant, beta (β), dynamically regularised using a go-seek function in Excel™, was added to all records before applying this transformation. This forward log-transformation for each data point i in the dataset can be written as:
The z-score of log-transformed (scaled) HSE content which is a metric that indicates how many standard deviations a data point is from the sample’s mean, assuming a gaussian distribution was calculated on the log-transformed data. This makes z-score a parametric method. When computing the z-score for each sample on the data set, no threshold was specified in order to avoid capping data on qualitative grounds. Using z-scores and normality tests, we scrubbed the datasets for significant outliers, here defined as any data points that are more than 4 standard deviations outside of the population mean. Estimation of the concentration of HSE mean, x̅, from the log-normal transformed data was done by calculating the log-variance, \( {s}_k^2 \), and making use of exponential function of Γi as illustrated in Eq. 2.
All the Au and PGE data were processed using Eqs. 1 and 2 and the variance of the back-transformed data was used to measure the spread between concentrations in each shale unit. For example, if a specific shale unit Au (ppm) mean is 2 and the variance is 0.002, the values are reported as x̅ = 2 ± 0.22. Graphical representation of outlier treatment is presented in Fig. 11.
Geochemistry
Major elements
We examined two Barberton Supergroup shale formations, namely the Sheba Formation of the Fig Tree Group, and the Clutha Formation of the Moodies Group. The mean SiO2/Al2O3 in the Fig Tree Group is x̅ = 4.60 (range 2.81–7.04) very similar to x̅ = 4.66 obtained for the Moodies Group (range 2.57–8.12). The Fe2O3/MgO of the Fig Tree Group (x̅ = 1.54, range 1.22–1.91) is slightly lower compared to that of the Moodies Group (x̅ = 1.29–11.65). The Ti content of the Fig Tree Group (x̅ = 0.59, range 0.29–0.74) is similar to that of the Moodies Group shales (x̅ = 0.57, range 0.22–0.91). Aluminium and Ti are stable or residual elements during chemical weathering, thus a positive correlation between Al and Ti indicate that weathering was an important control in the source area (Taylor and McLennan 1985). The Pearson correlation coefficient for TiO2-Al2O3 in the Fig Tree Group is +0.29, whereas in the Moodies Group it is as high as +0.80. The K2O content of marine shale is primarily a function of the clay mineral content, commonly illite in shale units. The Al2O3-K2O Pearson correlation coefficients of the studied shales in the Fig Tree and Moodies groups are +0.64 and + 0.45, respectively, and the K2O/Al2O3 ratios in the Fig Tree and Moodies groups are 0.21 and 0.23, respectively. Our results are in good agreement with those obtained in an independent study of the two groups, but with samples from different localities and formations (Toulkeridis et al. 2015).
The main chemical components in the studied Witwatersrand Supergroup shales show significant variations in concentration between individual stratigraphic units. The mean SiO2/Al2O3 of the Roodepoort Formation is x̅ = 3.95 (range 3.42–5.10), whereas the ratio for the Booysens Formation is x̅ = 2.98 (range 2.24–4.12, Western Areas goldfield) and x̅ = 3.01 (range 2.24–4.10, Welkom goldfield). The Pearson correlation coefficient for TiO2-Al2O3 in the Roodepoort Formation is +0.40, whereas in the Booysens Formation it is as high as +0.95. Expectedly, a strong positive correlation exists between Al2O3 and K2O (r = +0.80 in the Roodepoort Formation and + 0.91 in the Booysens Formation), with a K2O/Al2O3 of 0.30 (Nwaila et al. 2017).
The mean SiO2/Al2O3 of the Black Reef shales is 4.58 (range 3.46–5.23), whereas the mean K2O/Al2O3 is 0.28 (range 0.26–0.29). The Black Reef Formation shales display a strong positive correlation between TiO2 and Al2O3 (r = +0.96) and between Al2O3 and K2O (r = +0.97) and a strong negative correlation between SiO2 and K2O (r = −0.96). There is also a positive correlation between TiO2 and Fe2O3, MgO, MnO, and CaO, whereas CaO positively correlates with Fe2O3, MgO, and MnO.
The Silverton Formation shales of the upper Pretoria Group are divided into two subtypes based on stratigraphic depth: the upper shale unit (0–300 m) and lower shale unit (301–750 m). The shales’ SiO2 content ranges from 50.90 to 61.77 wt%, with a mean of 57.45 wt%. The mean Fe2O3 content of the Pretoria Group is with 5.66 wt% higher than that of PAAS.
Trace elements
Noteworthy are enrichments in Cr and Ni relative to PAAS and, to a lesser extent, Co in the Barberton Supergroup shales. In the shales of the Fig Tree and Moodies groups, the Cr contents mean are 874 ppm and 861 ppm, respectively, which is distinctly higher than in PAAS (110 ppm). Similarly, the mean Ni content in shales of the Fig Tree and Moodies groups are 343 and 351 ppm, respectively, also an order of magnitude higher than in PAAS (23 ppm). The Co contents in the same shale units are x̅ = 66 ppm and x̅ = 74 ppm, respectively, which is significantly above the PAAS value of 23 ppm. Shales from the Fig Tree and Moodies groups are depleted in total REE relative to PAAS (Fig. 2). In both groups, the fractionation behaviour between the REE is, on average, similar but varies between individual samples. Overall, these shales reveal fairly uniform REE patterns, with low total REE and LaN/YbN and positive Eu anomalies relative to PAAS. The shales from the Booysens Formation in the Welkom goldfield are enriched in REE compared to those from the same formation in the Western Areas goldfield and the Roodepoort Formation in the Carletonville goldfield (Fig. 2). A slightly positive Eu anomaly is noted for all Witwatersrand shales relative to PAAS (Eu* = 1.24).
Average (x̅) rare earth element concentrations of marine shales of the Barberton, Witwatersrand, and Transvaal supergroups, normalised against the Post-Archaean Australian shale composite (PAAS, normalisation values after Taylor and McLennan 1985). Coloured bands represent the range of data for the respective sample groups
The Black Reef Formation shales have slightly higher Zr/Y (x̅ = 10.45 ppm) and Zr/Nb (x̅ = 20 ppm) than PAAS. A strong positive correlation exists between the following elements: Ta–Nb (r = 0.97), Y–Nb (r = +0.88), and Zr–Hf (r = 1).
The Black Reef Formation shales have a low REE content when compared to PAAS (Fig. 2), likely due to their dilution with quartz as evident from very high SiO2 contents. The Silverton Formation shales of the Pretoria Group are depleted in Ba and especially Sr, and enriched in Co when compared to PAAS, whereas the content of all other elements is similar to the PAAS reference. A strong positive correlation exists between the following pairs of elements: Nb–Ga (r = +0.89), Nb–Hf (r = 0.7), Zr–Ga (r = 0.69), Zr–Hf (r = 1), Zr–Nb (r = 0.68), Ta–Ga (r = 0.84), and Ta–Nb (r = 0.94).
Gold and platinum group elements
Barberton Supergroup
The Au content of the Fig Tree Group shales ranges from 2 to 18 ppb (Fig. 3a), with x̅ = 4.61 and a variance of ±2.1 ppb. The Pt concentration in the same shale ranges from 2 to 8.5 ppb, with x̅ = 6.6 ± 0.02 ppb (Fig. 3a). Their Pd concentration is between 2 and 4 ppb, with x̅ = 3.7 ± 0.03 ppb (Fig. 3a). Other PGEs (Ir, Os, and Rh) in the Barberton Supergroup shales have concentrations below their detection limits (0.5 ppb), whereas the Ru content is within a very narrow range (1–3 ppb, reaching a maximum of 2 ppb in the Fig Tree Group and 3 ppb in the Moodies Group shales). The Au content of the Moodies Group shales ranges from 2 to 13 ppb, with x̅ = 2.23 ± 0.70 ppb (Fig. 3b). In contrast, its PGE content is less variable with no outliers. The Pt and Pd concentration in these shales range from 2 to 6 ppb, with little variation. In general, except for the Au outliers, the Moodies Group shales have similar HSE compositional patterns as the Fig Tree Group shales (Fig. 3). The TOC and TS content are generally low with x̅ = 0.40 wt% TOC and x̅ = 0.01 wt% TS (i.e. 0.02 % pyrite) in the Fig Tree Group shale, and x̅ = 0.51 wt% TOC and x̅ = 0.02 wt% TS in Moodies Group shale.
Gold and platinum group element concentrations in marine shales of the Fig Tree Group (a) and Moodies Group (b); other examples of such profiles through shale sequences of the Barberton Supergroup are given in Supplementary data S1
Witwatersrand Supergroup
The Au content of the Roodepoort Formation shale varies significantly, both vertically and laterally, and is x̅ = 6.57 ± 1.76 ppb (Fig. 4). The Pd and Pt concentrations in the same samples are x̅ = 4.20 ± 0.02 and x̅ = 3.97 ± 0.02 ppb, respectively, and are less variable both vertically and laterally (Fig. 4). Moreover, positive anomalies in Au content were noted in numerous samples of the Roodepoort Formation. A detailed Au concentration profile for the Roodepoort Formation obtained on drill core D5P03463 is provided in Fig. 4a. As evident from this figure, the Au concentration is uniform throughout (mostly between 3 and 7 ppb, except for a few outliers), reaching as much as 94 ppb at various stratigraphic depths, a phenomenon also revealed by data from other drill cores (Fig. 4b and c). The Pd and Pt concentrations range from 2 ppb to 8 and 7 ppb, respectively, throughout the Roodepoort Formation. No significant Pd outliers were noted in the sampled units, including those that are seemingly altered. The Roodepoort Formation shale samples contain, on x̅ = 0.12 wt% TOC and x̅ = 0.42 wt% TS (i.e. ~1 % pyrite).
The Booysens Formation was sampled in both the Welkom (Fig. 5a) and Western Areas goldfields (Fig. 5b). The Au content of these shale samples ranges from 6 to 26 ppb in the Welkom goldfield and 0.50 to 5 ppb in the Western Areas goldfield. The Pt and Pd content in both goldfields are similar and range from 3 to 5 (Fig. 5a) and from 6 to 13 ppb (Fig. 5b), respectively. Iridium, Os, and Rh concentrations in these shales are below their respective detection limits. The Ru concentrations are within 1–2 ppb across all assayed Witwatersrand shales, with a few samples having returned slightly higher values of up to 4 ppb. The Booysens Formation contains x̅ = 0.20 wt% TOC and x̅ = 0.09 wt% TS. There is no correlation between TOC, TS and Au (Fig. 6).
Geochemical plots for marine shales of the Barberton, Witwatersrand and Transvaal supergroups showing correlations of Au (ppb) with Ni (ppm), U (ppm), total organic carbon (TOC) (wt%) and total sulphur (TS) (wt%). See Fig. 2 for number of samples (N)
Transvaal Supergroup
The Au content of the Black Reef Formation shale ranges from 2 to 5.5 ppb, that of Pd from 9 to 10 ppb and that of Pt from 5 to 6 ppb (Fig. 7). All other PGE concentrations were below the detection limits, except for Ru (1–2 ppb). In general, the Pd and Pt concentrations are consistently and markedly higher than those of Au. In contrast to all other studied shale units, the Black Reef Formation shows a positive correlation between Au and TS (N = 4), and no correlation between Au and TOC (Fig. 6). The Black Reef Formation shale contains x̅ = 0.80 wt% TOC and x̅ = 0.04 wt% TS.
Despite the considerable thickness of the Pretoria Group, the Au concentrations in shale samples from a wide range of stratigraphic depths are all similar. The Au content of the upper Pretoria Group marine shales ranges from 1 to 5 ppb. In the lower shale unit, the Au content is between 1 and 3 ppb, with outliers reaching 7 ppb in drill core DP22 (Fig. 8a). The Au outliers occur close to lithological contacts with quartzite or siltstone, where some post-depositional fluid flow could have been possible. The Pd concentrations in the upper Pretoria Group shales range from 1 to 3 ppb. For the lower shale unit, the Pd content is highly variable, between 0.5 and 8.5 ppb, with the widest range present in drill core DP23 (Fig. 8b). Both Au and Pd outliers are present between 500 and 650 m in drill cores DP22 and DP23 (Fig. 8). In drill core DP22 the Pd concentration is between 1 and 2 ppb. Generally, the Pd content does not display regional systematic variation, although clustered, systematic increases and decreases are noted throughout the DP22 and DP23 drill cores. The Pt concentrations of the upper Pretoria Group marine shales range from 1 to 3 ppb (Fig. 8), those of the lower shale unit are uniform in drill core DP22 (Pd = 1 ppb) but variable in drill core DP23 (0.5–4 ppb). Elevated Pt content was noted at depths similar to those of the Au and Pd outliers (i.e. 500 m and 650 m, respectively). Drill core DP22 exhibits a progressive increase in Pt from the lower shale unit to the upper shale unit. In contrast, drill core DP23 displays a repetitive cycle of localised Pt increases and decreases from the lower shale unit to the upper shale unit. Other PGE (Ir, Os, and Rh) concentrations in these shales are below the detection limits, except for a few outliers of as much as 4 ppb. The Pretoria Group shales contain x̅ = 0.39 wt% TOC and 0.03 wt% TS.
Discussion
Sediment provenance
Certain trace elements especially REE and high field strength elements are regarded as good indicators of source rock composition (Taylor and McLennan 1985). Relatively immobile elements are mainly adsorbed onto clays that are held in suspension prior to sites of mud deposition (Taylor and McLennan 1985). This is supported by the fact that the REE, high field strength elements, and transition metal concentrations are significantly higher in the suspended load than in the dissolved load in major rivers of the world (Goldstein and Jacobsen 1988; Dupré et al. 1996).
A recent study by Nwaila et al. (2017) revealed that in the absence of biological Ni transfer in the ocean, Ni and Cr abundances should mirror the proportion of (ultra)mafic source rocks if neither of the elements were subjected to post-depositional mobility. In the marine shales studied here, the Ni and Cr abundances are high in both the Fig Tree (Ni = 59–593 ppm; Cr = 23–1277 ppm) and Moodies groups (Ni = 68–543 ppm and Cr = 55–1549 ppm) when compared to PAAS (Fig. 9). These values are among the highest recorded for Archaean shales and can be attributed to the high abundance of (ultra)mafic source rocks and the scavenging of the transition elements by clay-sized particles (McLennan 1993; Toulkeridis et al. 2015). These Ni and Cr concentrations represent values several times as high as those found in post-Archaean shales (Condie 1993). As expected, Ni is negatively correlated with SiO2 and positively correlated with MgO in both the Fig Tree and Moodies groups. Although the absolute abundances differ, the shale Cr/Ni ratios are very consistent (2.36 ± 0.47 and 2.57 ± 0.62), and similar to that of Archaean komatiite (Cr/Ni = 2.4; Condie 1993). The Cr/Ni ratio in the shale, therefore, records the source material’s geochemical signal without significant fractionation (Durrheim and Mooney 1994). This results in high Cr/V and Ni/Co and low V/Ni for both the Fig Tree and Moodies groups. In addition, both Cr and Ni are positively correlated with Mg, Sc, and V, emphasizing the dominance of (ultra)mafic rocks in the source area.
A mixed felsic/mafic hinterland is suggested for the Roodepoort Formation shale (Fig. 9). A strong positive correlation between Ni and Cr (r = 0.84) suggests that the two elements remained relatively immobile after sediment deposition. A similarly positive Ni–Cr correlation of r = 0.91 is also noted for the Booysens Formation shales in different goldfields. However, the absolute concentrations of the same elements vary significantly up-section, reflecting a progressive increase in felsic/mafic source-rock proportions during sedimentation (Nwaila et al. 2017). In the Black Reef Formation, the Ni and Cr values suggest sediment input from mixed source rocks (Fig. 9). The Pretoria Group has relatively low abundances of Ni and Cr from which a predominantly felsic provenance can be deduced (Fig. 9).
The Th/Sc ratio can also be useful for provenance studies, due to felsic rocks’ enrichment in Th-bearing minerals and the affinity of Sc for mafic compositions (Fig. 10). Low Th/Sc (<1) indicates a mafic source, which is usually enriched in incompatible elements such as Sc, whereas the incompatibility of Th results in higher concentrations in well-differentiated felsic rocks (Th/Sc ± 1; Taylor and McLennan 1985). Thus, the Th/Sc is an effective indicator of chemical source rock signature, and the Zr/Sc is a useful index of zircon enrichment (McLennan 1993). Therefore, the Th/Sc versus Zr/Sc plot can be used to evaluate both the source-rock composition and the degree of sedimentary sorting and recycling. The Barberton Supergroup shales exhibit a linear relationship between the two ratios, with a greater dispersion of shales toward higher Th/Sc and Zr/Sc. This trend indicates a consistent provenance and compositional variation without zircon enrichment, suggesting that the studied shales from the Fig Tree and Moodies groups did not undergo intrabasinal recycling. Marine shales of the Booysens Formation indicate more diverse source rocks when compared to those of the Roodepoort Formation. The shale samples display a wide range of Th/Sc (0.1–2.0), reflecting highly variable proportions of felsic versus mafic rocks in the source area, a phenomenon mirrored by the strong variation in Zr/Cr.
Distribution of gold and platinum group elements
The HSE content of the studied shale units in the Kaapvaal Craton varies significantly, both within as well as between different stratigraphic units (Fig. 11). This may be due to a variety of reasons such as differences in HSE endowment in the respective hinterlands and mode of HSE transport to and incorporation into shale. The Au-Al2O3 correlation coefficient of the studied shales has a narrow range (Fig. 12) except for few outliers especially close to the shale-siltstone intercalations. This correlation suggests that at least some Au was transported as colloidal particles that were attached to clay minerals during transport and/or sedimentation.
Secular variation in the concentrations of Au, Pt, and Pd in marine shales of the Kaapvaal Craton. Fm. = Formation; GF = Goldfield. For the various features of the whisker-boxplot. See Fig. 2 for number of samples (N)
Correlation between Au (ppb) content and Al2O3 (wt%), indicating changes in clay material input. See Fig. 2 for number of samples (N)
Geochemical data of marine shales of the Barberton, Witwatersrand and Transvaal supergroups all indicate intense source area weathering (Fig. S1). If the gold in the studied marine shales had been derived from high rates of erosion of specific source rocks due to chemical weathering (Fig. S1), the studied marine shales should have geochemical variations that correlate directly with Au abundances in the respective source rocks. The Kaapvaal Craton has several granitoid-greenstone terranes from which the shales were sourced. Pitcairn (2011) estimated the crustal Au average in Archaean rocks as between 0.5 and 5 ppb in unmineralised igneous, sedimentary, and metamorphic rocks. However, Hofmann et al. (2017) showed that submarine alteration led to a reduction of gold content during the Palaeoarchaean. Compared to these values, some of the Au concentrations obtained in this study, particularly those of the Mesoarchaean Central Rand Group, are higher, at least for the Archaean portions of the Kaapvaal Craton. This could be due to either a higher Au flux into the Archaean ocean or a more mafic nature of the hinterland. Considering that some of the Au-rich shales, e.g. Booysens Formation shale in the Welkom goldfield, were derived from felsic source rocks (Nwaila et al. 2017), we favour the former explanation for the elevated Au content in the marine shales.
The Black Reef Formation shales differ from all others by having much higher TOC content (0.52–1.10 wt%) and stronger enrichment in Pd and Pt than in Au. They display a high positive correlation (>0.80) between Au and TS in 4 samples (Fig. 6d). These differences to the other studied shales may be due to differences in the style of post-depositional alteration. The Black Reef Formation experienced intense, post-depositional hydrothermal alteration by circulating aqueous-carbonic fluids (Fuchs et al. 2016b) that led to abundant pyrobitumen (hence the term “Black Reef”). The Pt and Pd absorption on organic matter best explains the elevated HSE. We suggest that the specific fluid chemistry enabled higher concentrations of HSE, especially PGE, thus explaining the anomalous (with respect to other Archaean shales of the Kaapvaal Craton) PGE content in the Black Reef Formation. In contrast, all the marine shale units in the Pretoria Group experienced only diagenesis (Coetzee et al. 2006) with little alteration and HSE mobility.
Potential sources and differences in HSE endowment in the respective hinterlands
Apart from the extensive literature on highly metalliferous Phanerozoic black shales (e.g. Johnson et al. 2017), our knowledge of the source of HSE in Archaean and Palaeoproterozoic marine shales remains limited. The Kaapvaal Craton has a number of greenstone belts, with some of them containing HSE background concentrations above today’s crustal average (Saager and Meyer 1984; Pitcairn 2011). In this instance, greenstone belts should provide the principal source of HSE in the sedimentary rocks that were derived from them. This is particularly evident in the case of the Kaapvaal Craton, which contains numerous granitoid-greenstone rocks (i.e. the Barberton, Sutherland, Rhenosterkoppies, Muldersdrift, Pietersburg, Giyani, Murchison, Kraaipan, and Amalia greenstone belts). As far as they have been analysed, they contain Au contents ranging from 0.5 to 5 ppb on average with outliers of as much as 667 ppb (Saager and Meyer 1984; Maier et al. 2009). These outliers probably represent secondary concentrations and therefore cannot be used to characterise the primary rock composition. Our sediment provenance analysis shows that detritus for the marine shale units was derived from a mixed granite-greenstone-like source, with a large proportion of mafic to ultramafic source rocks.
Recent studies suggested an increased capacity of Archaean river water to transport Au in dissolved form, likely as a sulphide complex (Frimmel 2014; Frimmel and Hennigh 2015; Heinrich 2015). In coastal and shallow marine environments, microbial colonies might have trapped a considerable amount of that gold, be it by oxidation as suggested by Frimmel (2014) and Frimmel and Hennigh (2015), or by reduction, as suggested by Hallbauer (1975), Horscroft et al. (2011), and, more recently, Heinrich (2015). While microbial gold fixation can explain the exceptional gold endowment of kerogen seams and quartz-pebble conglomerates in the Witwatersrand reefs (Minter et al. 1993), this does not necessarily explain all the Au content in marine shales. Although microbial gold fixation is more likely to occur in the low-energy environment of shale deposition, a lack of correlation between HSE and TOC in the studied marine shale samples is noteworthy as it implies a gold concentration process that is not fully dependent on microbial activity. Despite microbial activity generally having taken place on coastal plains and flood plains, where HSE fixation would occur, followed by the transfer of some of the carbon to the deeper water, much of carbon sourced from such surfaces would have decomposed during the formation of sedimentary pyrite and thus does not explain the HSE content to levels measured in the studied marine shales.
Irrespective of the gold source, the question of how Au (and other HSE) became incorporated into the Archaean and Palaeoproterozoic marine shales needs to be addressed. Ketris and Yudovich (2009) calculated an average global gold concentration in carbon-rich black shales of 7 ± 1 ppb, which is much higher than in other sedimentary rocks. Although this average is based on some 9120 analyses, most of the samples are Phanerozoic in age and most are metal-enriched black shales, e.g. the Kimkan and Sutyr’ units of the Bureya massif (southern Far East, Russia; Khanchuk et al. 2013). This average confirms the already well-established notion that black shales are, in general, enriched in a range of metals, including HSE (e.g. Meyers et al. 1992; Pašava et al. 2013).
The higher concentrations of Au and PGE in black shales are thought to be due to organic complexation of Au and PGE from seawater in anoxic organic-rich mud which is eventually incorporated into diagenetic sulphide minerals (Helz et al. 1996). More recently, Johnson et al. (2017) demonstrated that the increasing sequestration of carbon and sulphur during high periods of black shale formation resulted from high atmospheric O2. Such atmospheric conditions did not exist in the Mesoarchaean (e.g. Holland 1962; Burron et al. 2018), and marine shales with high TOC are effectively rare from this eon. The formation of biogenic pyrite in organic-rich sediments can be traced back well into the Archaean. Evidence for microbial sulphate reduction has been described from rocks as old as 3.4 Ga (e.g., Ohmoto et al. 1993). Majority of the original synsedimentary TOC content in Archean rocks have been subjected to varying conditions of metamorphism coupled with the decomposition of carbon aided by the Archaean atmospheric conditions and the composition of seawater. The preservation efficiency of original TOC produced in surface waters in Phanerozoic anoxic basins (that is, where preservation efficiency is highest in the modern ocean) are of the order of 1–2 % (Thunell et al. 2000) whereas this value is as low as <0.1 % for the Archaean environments. Thus, Phanerozoic black shales may be a poor analogue for the reconstruction of gold-enrichment in Archaean shales due to significant differences in the synsedimentary TOC content.
Sedimentary pyrite is known to contain appreciable amounts of gold (up to several tens of ppm) and other metals (Hallbauer 1986; Guy et al. 2010; Large et al. 2015; Agangi et al. 2015). In the Witwatersrand Supergroup, gold in sedimentary pyrite was initially regarded as secondary because of infiltrating Au-bearing metamorphic fluids (Koppel and Saager 1974). However, a review of pyrite paragenetic sequence led some researchers to suggest that gold in sedimentary pyrite is syngenetic (Hallbauer and Barton 1987; Robb et al. 1990). More recently, Large et al. (2015) proposed that the gold in sedimentary pyrite was formed by adsorption from seawater during sedimentary pyrite growth in an anoxic environment.
To evaluate the contribution of pyrite to the HSE content in marine shales, we applied a mass-balance calculation based on the abundance and composition of synsedimentary marine pyrite (Large et al. 2015; Agangi et al. 2015). For illustrative purposes, we selected the Booysens Formation marine shales (Welkom goldfield). Marine shales of the Booysens Formation are interpreted to have been deposited in a restricted basin (i.e. flooded foreland basin; Kositcin and Krapež 2004). This shale unit contains the highest HSE content of all studied units. The average Au concentration (90 ppb) in a typical sedimentary pyrite was calculated from data reported by Large et al. (2015). Marine shales of the Booysens formation have a modal pyrite proportion of <3 vol.%, obtained using optical microscopy and X-ray powder diffraction (Nwaila et al. 2017). On a local scale, the contribution of pyrite may appear to be more plausible, however, modelling of the volumetric pyrite/shale ratio indicates that the total contribution of gold in marine shales of the Booysens Formation from Archaean sedimentary pyrite is 2.7 ppb. This value is two to three times lower than the Au concentrations (>6 ppb) measured in the Booysens Formation (Welkom goldfield). While sedimentary pyrite certainly contributed to the overall HSE endowment in the marine shales, its modal proportion is too small to solely account for the total background colloidal gold content. Similarly, sedimentary pyrite alone does not explain the amount of Au noted in the Pretoria Group shales (2.65 ppb) because of a generally low pyrite content in these shales (<0.5 vol.%).
The lack of significant concentrations of organic matter and lower atmospheric O2 in the Archaean (e.g. Holland 1962; Lyons et al. 2014) or the inability of sedimentary pyrite to account for all the gold found in these shales calls for an alternative gold-concentration mechanism, especially for colloidal gold particles that are purported to occur in marine shales. To determine whether gold entered marine mud as colloidal gold or through precipitation from seawater (or other fluid), we correlated Au with ratios of elements typical of detrital minerals derived from felsic and mafic sources. The Booysens Formation, which contains the highest of all marine shale Au concentrations, serves as an example. Zirconium and Th are taken as proxies of detrital material from felsic source rocks, whereas Ni and Sc serve as proxies for mafic source rocks. Plotting Au against Zr/Ni and Th/Sc reveals a distinct negative correlation (Fig. S2), suggesting that a significant proportion of Au may have been transported as colloidal Au or that it was sourced mainly from mafic source rocks such as greenstones. As suggested by Nwaila et al. (2017) Ni in the Archaean marine shales was sourced from mafic/ultramafic rocks in the hinterland.
Gold residence time in the modern ocean environment is estimated to be approximately 1000 years (Falkner and Edmond 1990), which is equivalent to the mixing time of ocean water. It would, therefore, be intuitive to expect a uniform distribution of Au throughout the open ocean (Large et al. 2015). However, Au concentrations of up to an order of magnitude higher have been measured in seawater close to continental margins and large river mouths (Nekrasov 1996), with lower values being recorded in the open oceans (Large et al. 2015). Some of the modern oceans (e.g. the Arctic Ocean) that are fed by rivers (e.g. Siberia’s Lena River) have high concentrations of gold (60 ppb). This is likely due to sourcing of Au from active mining areas which is transported as suspended particles and deposited into the ocean (Falkner and Edmond 1990).
If some of the Au in the Archaean marine shales entered the ocean in the form of colloidal gold, finely dispersed sediments floating in marine environments may have gold adsorbed to clay-sized particles. Colloidal gold together with clay would eventually sink and settle on the seafloor (Weitz et al. 1984). In Phanerozoic oceans with sufficient natural flocculants, such as organic carbon and sulphate, this process can occur rapidly (provided that there is sufficient HSE) as they tend to have a great fixation affinity for HSE (Southam et al. 2009). This principle has been applied both experimentally and practically to extract gold in metallurgical plants (e.g. carbon in pulp or carbon in leach processes; Wadnerkar et al. 2015) and to remove mud and its associated organic and inorganic compounds in water treatment plants (Tzoupanos and Zouboulis 2008).
Sediment trap experiments have been used to test aggregation and sinking rates of clay-sized particles. The results suggest that for sediments to sink, they must first coalesce and bond in less turbulent marine environments (Gardner 1980). Therefore, a marine setting provides an ideal environment for colloidal gold particles and clay-sized sediments to undergo mechanical coagulation and aggregation prior to sinking and subsequent settling (Trinh et al. 2009). The concentration of gold during marine sedimentation may be more efficient where the clastic sediment load that carries colloidal gold is held in suspension for a longer time than the actual sedimentation rate, and/or where the background concentration of gold is higher.
Recent studies on the origin of the Witwatersrand gold deposits have suggested very high fluvial Au flux off the Archean land surface around 2.94–2.87 Ga, with the maximum peak occurring during the deposition of the Central Rand Group sediments (Frimmel 2014; Heinrich 2015). A peak in marine synsedimentary Au–enrichment at Central Rand times correlates with the period of maximum gold concentration in fluvial to fluviodeltaic conglomerates and supports the notion of coeval elevated Au flux off the Archaean hinterland. Published palaeoclimate data indicate that during West Rand Group times, the climate was cold, at least on the Kaapvaal Craton, as indicated by the presence of glaciogenic diamictite, whereas Central Rand Group times were marked by warm climatic conditions (Frimmel 2005, 2018), enabling higher chemical weathering rates and consequently higher capacity for Au flux off the land surface (Frimmel 2014). This might well explain the higher Au content of the Booysens Formation shale and the overall much higher gold endowment of the Central Rand compared to the West Rand Group (Frimmel 2018).
Conclusions
Marine shales in the supracrustal successions of the Kaapvaal Craton showcase two important aspects of the Mesoarchaean and Palaeoproterozoic geology; (a) nature of the source rocks representing granitoid-greenstone hinterland and, (b) distribution of gold and PGEs in marine sediments. A mixture of source rocks for the studied marine shales and very intense chemical weathering in the source rock area are inferred from elemental ratios (e.g. Ni/Cr, Th/Sc and Zr/Sc) and various indices of chemical variability and chemical weathering. Microbial communities inhabited the Archaean surface environments where they played a role in the formation of sedimentary pyrite and trapping some of the HSE. Results from this study showed that there is no correlation between Au and total organic carbon for most of the Kaapvaal Craton marine shales. Based on modal abundance, sedimentary pyrite also played a major role in contributing to the overall gold content in the Kaapvaal Craton, however, the lack of correlation between gold and total sulphur content suggests that some of the gold (and PGE) in the Archaean and Proterozoic oceans was mechanically or colloidally transferred into the ocean. There are notable HSE endowment differences in some of the marine shales, such as those of the Witwatersrand Supergroup, e.g. Roodepoort Formation in the upper West Rand Group and the Booysens Formation in the middle Central Rand Group. They are likely to reflect secular variations in gold content of seawater, which are probably related to a combination of surface hydro- and biochemical conditions, source rock lithology and climate change, resulting in a global crustal gold peak at around 2.9 Ga (Frimmel 2018).
References
Abou-Shakra FR (2013) Chapter 12 - biomedical applications of inductively coupled plasma mass spectrometry (ICP–MS) as an element specific detector for chromatographic separations. In: Wilson ID (ed) Handbook of analytical separations, Bioanalytical separations, vol 4, pp 351–371
Agangi A, Hofmann A, Rollion-Bard C, Marin-Carbonne J, Cavalazzi B, Large R, Meffre S (2015) Gold accumulation in the Archaean Witwatersrand Basin, South Africa—evidence from concentrically laminated pyrite. Earth Sci Rev 140:27–53
Armstrong RA, Compston W, De Wit MJ, Williams IS (1990) The stratigraphy of the 3.5–3.2 Ga Barberton Greenstone Belt revisited: a single zircon ion microprobe rare study. Earth Planet Sci Lett 101:90–106
Beukes NJ (1995) Stratigraphy and basin analyses of the West Rand Group with special reference to prospective areas for placer gold deposits. Johannesburg, Rand Afrikaans University Geology Department, Report, 117 pp
Burron I, da Costa G, Sharpe R, Fayek M, Gauert C, Hofmann A (2018) 3.2 Ga detrital uraninite in the Witwatersrand Basin, South Africa: evidence of a reducing Archean atmosphere. Geol Soc Am Bull 46:295–298
Byerly GR, Kröner A, Lowe DR, Todt W, Walsh MM (1996) Prolonged magmatism and time constraints for sediment deposition in the early Archaean Barberton greenstone belt: evidence from the Upper Onverwacht and Fig Tree groups. Precambrian Res 78:125–138
Coetzee LL, Beukes NJ, Gutzmer J, Kakegawa T (2006) Links of organic carbon cycling and burial to depositional depth gradients and establishment of a snowball Earth at 2.3 Ga. Evidence from the Timeball Hill Formation, Transvaal Supergroup, South Africa. S Afr J Geol 109:109–122
Condie KC (1993) Chemical composition and evolution of the upper continental crust: contrasting results from surface samples and shales. Chem Geol 104:1–37
Coward MP, Spencer RM, Spencer CE (1995) Development of the Witwatersrand Basin, South Africa. In: Coward, M. P, Ries, A.CA. (Eds.), Early Precambrian processes. Geological Society of London Special Publications, London, pp 243–269
Dankert BT, Hein KAA (2010) Evaluating the structural character and tectonic history of the Witwatersrand Basin. Precambrian Res 177:1–22
Dupré B, Gaillardet J, Rousseau D, Allégre C (1996) Major and trace elements of river-borne material: the Congo basin. Geochim Cosmochim Acta 60:1301–1321
Durrheim RJ, Mooney WD (1994) Evolution of the Precambrian lithosphere; seismological and geochemical constraints. J Geophys Res 99:15359–15374
Eriksson PG, Altermann W, Hartzer F (2006) The Transvaal Supergroup and its precursors. In: Johnson, M. R., Anhaeusser, C. R., Thomas, R. (Eds.), The geology of South Africa. Geological Society of South Africa and Council of Geoscience, Pretoria, pp 237–260
Falkner KK, Edmond JM (1990) Gold in seawater. Earth Planet Sci Lett 98:208–221
Frimmel HE (1994) Metamorphism of Witwatersrand Gold. Explor Min Geol 3:357–370
Frimmel HE (2005) Archaean atmospheric evolution: evidence from the Witwatersrand gold fields, South Africa. Earth-Sci Rev 70:1–46
Frimmel HE (2014) A giant Mesoarchaean crustal gold–enrichment episode: possible causes and consequences for exploration. Econ Geol Spec Pub 18:209–234
Frimmel HE (2018) Episodic concentration of gold to ore grade through Earth's history. Earth-Sci Rev 180:148–158
Frimmel HE, Hennigh Q (2015) First whiffs of atmospheric oxygen triggered onset of crustal gold cycle. Mineral Deposita 50:5–23
Frimmel HE, Minter WEL (2002) Recent developments concerning the geological history and genesis of the Witwatersrand gold deposits, South Africa. Econ Geol Spec Pub 9:17–45
Frimmel HE, Groves DI, Kirk J, Ruiz J, Chesley J, Minter WEL (2005) The formation and preservation of the Witwatersrand goldfields, the largest gold province in the world. In: Hedenquist JW, Thomson JFH, Goldfarb RJ (eds) Economic Geology 100th Anniversary Volume. Society of Economic Geologists, Littleton, pp 769–797
Fuchs SHJ, Williams-Jones AE, Jackson SE, Przybylowicz WJ (2016a) Metal distribution in pyrobitumen of the carbon leader reef, Witwatersrand Supergroup, South Africa: evidence for liquid hydrocarbon ore fluids. Chem Geol 426:45–59
Fuchs S, Williams-Jones AE, Przybylowicz WJ (2016b) The origin of the gold and uranium ores of the black reef formation, Transvaal Supergroup, South Africa. Ore Geol Rev 72:149–164
Fuller AO, Camden-Smith P, Sprague ARG, Waters DJ, Willis J (1981) Geochemical signature of shales from the Witwatersrand Supergroup. S Afr J Sci 77:379–381
Gardner WD (1980) Sediment trap dynamics and calibration: a laboratory evaluation. J Mar Res 38:17–39
Goldberg ED (1987) Heavy metal analyses in the marine environment– approaches to quality control. Mar Chem 22:117–124
Goldstein SJ, Jacobsen S (1988) Rare earth elements in river waters. Earth Planet Sci Lett 89:35–47
Goovaerts P, Albuquerque MTD, Antunes IMHR (2016) A multivariate geostatistical methodology to delineate areas of potential interest for future sedimentary gold exploration. Math Geosci 48:921–939
Gumsley A, Stamsnijder J, Larsson E, Söderlund U, Naeraa T, de Kock MO, Ernst R (2018) The 2789–2782 Ma Klipriviersberg large igneous province: implications for the chrono-stratigraphy of the Ventersdorp Supergroup and the timing of Witwatersrand gold deposition. GeoCongress 2018, Geological Society of South Africa (GSSA), Abstract Book, p 133
Gutzmer J, Beukes NJ (1998) Earliest laterites and possible evidence for terrestrial vegetation in the early Proterozoic. Geology 26:263–266
Guy, BM (2012) Pyrite in the Mesoarchaean Witwatersrand Supergroup, South Africa. PhD thesis, University of Johannesburg, Auckland Park, pp 218–304
Guy BM, Beukes NJ, Gutzmer J (2010) Palaeoenvironmental controls on the texture and chemical composition of pyrite from non–conglomeratic sedimentary rocks of the Mesoarchaean Witwatersrand Supergroup, South Africa. S Afr J Geol 113:195–228
Halevy I, Bachan A (2017) The geologic history of seawater pH. Science 355:1069–1071
Hallbauer DK (1975) The plant origin of Witwatersrand carbon. Miner Sci Eng 7:111–131
Hallbauer DK (1986) The mineralogy and geochemistry of the Witwatersrand pyrite, gold, uraninite and carbonaceous matter. In: Anhaeusser, C. R., Maske, S. (Eds.), Mineral Deposits of Southern Africa. Geological Society of South Africa, pp 731–752
Hallbauer DK, Barton J (1987) The fossil gold placers of the Witwatersrand. Gold Bull 20:68–79
Hannah JL, Bekker A, Stein HJ, Markey RJ, Holland HD (2004) Primitive Os and 2316 Ma age for marine shale: implications for Paleoproterozoic glacial events and the rise of atmospheric oxygen. Earth Planet Sci Lett 225:43–52
Harmer RE, Armstrong RA (2000) New precise dates on the acid phase of the Bushveld and their implications. Abstract. Workshop on the Bushveld Complex, 18th-21st November 2000, Burgersfort. University of Witwatersrand, Johannesburg
Heinrich CA (2015) Witwatersrand gold deposits formed by volcanic rain, anoxic rivers and Archaean life. Nat Geosci 8:206–209
Helz GR, Miller CV, Charnock JM, Mosselmans JFW, Patrick RAD, Garner CD, Vaughan D (1996) Mechanisms of molybdenum removal from the sea and its concentration in black shales: EXAFS evidence. Geochim Cosmochim Acta 60:3631–3642
Heubeck C (2014) The Moodies Group (3.22–3.21 Ga), Barberton Greenstone Belt: a multidimensional archive of Archaean information. The Geological Society of America (GSA) Annual Meeting in Vancouver, British Columbia (19–22 October 2014) 85, p. 4
Heubeck C, Lowe DR (1994) Late syndepositional deformation and detachment tectonics in the Barberton Greenstone Belt, South Africa. Tectonics 13:1514–1536
Heubeck C, Engelhardt J, Byerly GR, Zeh A, Sell B, Luber T, Lowe DR (2013) Timing of deposition and deformation of the Moodies Group (Barberton Greenstone Belt, South Africa): Very-high-resolution of Archaean surface processes. Precambrian Res 231:236–262
Heubeck C, Blasing S, Grund M, Drabon N, Homann M, Nabhan S (2016) Geological constraints on Archaean (3.22 Ga) coastal-zone process from the Dycedale syncline, Barberton Greenstone Belt. S Afr J Geol 119:495–518
Hofmann A (2005) The geochemistry of sedimentary rocks from the Fig Tree Group, Barberton greenstone belt: implications for tectonic, hydrothermal and surface processes during mid-Archaean times. Precambrian Res 143:23–49
Hofmann A, Pitcairn I, Wilson A (2017) Gold mobility during Palaeoarchaean submarine alteration. Earth Planet Sci Lett 462:47–54
Holland HD (1962) Model for the evolution of the Earth’s atmosphere. In: Engel AEJ et al (eds) Petrologic Studies: a volume to honor A.F. Buddington. Geological Society of America, New York, pp 447–477
Horscroft FDM, Mossman DJ, Reimer TO, Hennigh Q (2011) Witwatersrand metallogenesis: the case for (modified) syngenesis. SEPM Spec Publ 101:75–95
Hronsky JMA, Groves DI, Loucks RR, Begg GC (2012) A unified model for gold mineralisation in accretionary orogens and implications for regional-scale exploration targeting methods. Mineral Deposita 47:339–358
Huffman EL, Clark JR, Yeager JR (1998) Gold analysis - fire assaying and alternative methods. Explor Min Geol 7:155–160
Hutchinson RW, Ridler RH, Suffel GG (1971) Metallogenic relationships in the Abitibi belt, Canada: a model for Archean metallogeny. CIM 74:106–115
Johnson SC, Large RR, Coveney RM, Kelley KD, Slack JF, Steadman JA, Gregory DD, Sack PJ, Meffre S (2017) Secular distribution of highly metalliferous black shales corresponds with peaks in past atmosphere oxygenation. Mineral Deposita 52:791–798
Kamo SL, Reimold WU, Krogh TE, Colliston WP (1996) A 2.023 Ga age for the Vredefort impact event and a first report of shock metamorphosed zircons in pseudotachylitic breccias and granophyre. Earth Planet Sci Lett 144:369–387
Ketris MP, Yudovich Y (2009) Estimations of Clarkes for carbonaceous bioliths: world averages for trace element contents in black shales and coals. Int J Coal Geol 78:135–148
Khanchuk A, Nevstruev VG, Berdnikov NV, Nachaev VP (2013) Petrochemical characteristics of carbonaceous shales in the eastern Bureya massif and their precious-metal mineralization. Russ Geol Geophys 54:627–636
Koide M, Goldberg ED, Niemeyer S, Gerlach D, Hodge V, Bertine KK, Padova A (1991) Osmium in marine sediment. Geochim Cosmochim Acta 55:1641–1648
Koppel VH, Saager R (1974) Lead isotope evidence on the detrital origin of Witwatersrand pyrites and its bearing on the provenance of the Witwatersrand gold. Econ Geol 69:318–331
Kositcin N, Krapež B (2004) SHRIMP U–Pb detrital zircon geochronology of the late Archaean Witwatersrand Basin of South Africa: relation between zircon provenance age spectra and basin evolution. Precambrian Res 129:141–168
Krige DG (1960) On the departure of ore value distributions from the log-normal model in south African gold mines. J South Afr Inst Min Metall 62:63–64
Krupp R, Oberthür T, Hirdes W (1994) The early Precambrian atmosphere and hydrosphere: thermodynamic constraints from mineral deposits. Econ Geol 89:1581–1598
Large RR, Bull SW, Maslennikov VV (2011) A carbonaceous sedimentary source-rock model for Carlin-type and orogenic gold deposits. Econ Geol 106:331–358
Large RR, Meffre S, Burnett R, Guy B, Bull S, Gilbert S, Goemann K, Danyushevsky L (2013) Evidence for an intrabasinal source and multiple concentration processes in the formation of the carbon leader reef, Witwatersrand Supergroup, South Africa. Econ Geol 108:1215–1241
Large RR, Gregory DD, Steadman JA, Tomkins AG, Lounejeva E, Danyushevsky LV, Halpin JA, Maslennikov V, Sack PJ, Mukherjee I, Berry R, Hickman A (2015) Gold in the oceans through time. Earth Planet Sci Lett 428:139–150
Lehmann B, Nägler TF, Holland HD, Wille M, Mao J, Pan J, Ma D, Dulski P (2007) Highly metalliferous carbonaceous shale and early Cambrian seawater. Geology 35:403–406
Lorand J-P, Luguet A, Alard O (2008) Platinum-group elements: a new set of key tracers for the earth’s interior. Elements 4:247–252
Lowe DR, Byerly GR (1999) Stratigraphy of the westcentral part of the Barberton Greenstone Belt, South Africa. In: Lowe DR, Byerly GR (eds) Geologic evolution of the Barberton Greenstone Belt, South Africa. Geological Society of America Special Papers, Boulder, pp 1–36
Lyons TW, Reinhard CT, Planavsky NJ (2014) The rise of oxygen in Earth’s early ocean and atmosphere. Nature 506:307–315
Maier WD, Barnes SJ, Campbell IH, Fiorentini ML, Peltonen P (2009) Progressive mixing of meteoritic veneer into the early Earth’s deep mantle. Nature 460:620–623
Mashio AS, Obata H, Gamo T (2017) Dissolved platinum concentrations in coastal seawater: Boso to Sanriku areas, Japan. Arch Environ Contam Toxicol 73:240
McCarthy TS (2006) The Witwatersrand Supergroup. In: Anhaeusser CR, Thomas RJ (eds) Johnson MR. The Geology of South Africa, Johannesburg, Geological Society of South Africa, pp 155–186
McDonald JH (2014) Handbook of biological statistics, 3rd edn. Sparky House Publishing, Baltimore, pp 140–144
McLennan SM (1993) Weathering and global denudation. J Geol 101:295–303
McLennan JA, Deutsch CV (2004) Conditional non-bias of geostatistical simulation for estimation of recoverable reserves. CIM Bull 97:68–72
Meyer FM, Robb LJ (1996) Geochemistry of black shales from the Chuniespoort Group, Transvaal sequence, South Africa. Geochim Cosmochim Acta 91:111–121
Meyer FM, Saager R (1985) The gold content of some Archaean rocks and their possible relationship to epigenetic gold-quartz vein deposits. Mineral Deposita 20:284–289
Meyers PA, Pratt LM, Nagy B (1992) Introduction to geochemistry of metalliferous black shales. Chem Geol 99:189–211
Minter WEL, Goedhart M, Knight J, Frimmel HE (1993) Morphology of Witwatersrand gold grains from the basal reef: evidence for their detrital origin. Econ Geol 88:237–248
Nekrasov IY (1996) Geochemistry, mineralogy and genesis of gold deposits. A.A. Balkema, Moscow, p 344
Nwaila G, Frimmel HE, Minter WEL (2017) Provenance and geochemical variations in shales of the Mesoarchaean Witwatersrand Supergroup. J Geol 125:399–422
Ohmoto H, Kakegawa T, Lowe DR (1993) 3.4-billion-year-old biogenic pyrites from Barberton, South Africa: sulfur isotope evidence. Science 262:555–557
Pašava J, Zaccarini F, Aiglsperger T, Vymazalová A (2013) Platinum–group elements (PGE) and their principal carriers in metal–rich black shales: an overview with new data from Mo–Ni–PGE black shales (Zunyi region, Guizhou Province, South China). J Geosci 58:213–220
Phillips NG, Law JDM (1994) Metamorphism of the Witwatersrand gold fields: a review. Ore Geol Rev 9:1–31
Phillips GN, Law JDM (2000) Witwatersrand gold fields: geology, genesis and exploration. Rev Econ Geol 13:439–500
Phillips GN, Powell R (2015) Hydrothermal alteration in the Witwatersrand goldfields. Ore Geol Rev 65:245–273
Pitcairn IK (2010) Source area processes and the distribution of orogenic gold deposits. In: Goldschmidt Conference, Knoxville, TN, USA, June, the University of Tennessee and Oak Ridge National Laboratory
Pitcairn IK (2011) Background concentrations of gold in different rock types. Appl Earth Sci 120:31–38
Pitcairn IK, Teagle DAH, Craw D, Olivo GR, Kerrich R, Brewer TS (2006) Sources of metals and fluids in orogenic gold deposits: insights from the Otago and Alpine schists, New Zealand. Econ Geol 101:1525–1546
Poujol M, Robb LJ, Anhaeusser CR, Gericke B (2003) A review of the geochronological constraints on the evolution of the Kaapvaal Craton, South Africa. Precambrian Res 127:181–213
Ridler RH (1970) Relationship of mineralization to volcanic stratigraphy in the Kirkland-Larder Lakes area, Ontario. Annual General Meeting (AGM) of the Geological Association of Canada 21:33–42
Robb LJ, Meyer FM (1995) The Witwatersrand Basin, South Africa: geological framework and mineralisation processes. Ore Geol Rev 10:67–94
Robb LJ, Meyer FM, Ferraz MF, Drennan GK (1990) The distribution of radioelements in Archaean granites of the Kaapvaal Craton, with implications for the source of uranium in the Witwatersrand Basin. S Afr J Geol 93:5–40
Robb LJ, Charlesworth EG, Drennan GR, Gibson RL, Tongu EL (1997) Tectono-metamorphic setting and paragenetic sequence of Au-U mineralisation in the Archaean Witwatersrand basin. South Africa: AJES 44:353–371
Rudnick RL, Gao S (2005) 3.01 – composition of the continental crust. In: Rudnick R (ed) Treatise on geochemistry. Elsevier, Amsterdam, pp 1–64
Saager R, Meyer M (1984) Gold distribution in Archaean granitoids and supracrustal rocks from South Africa: a comparison. In: Foster RP (ed) Gold. Geology Soca. Zimbabwe Special Publication. 1. Balkema, Rotterdam, pp 53–70
Schoene B, Dudas FOL, Bowring, SA, de Wit M (2009) Sm–Nd isotopic mapping of lithospheric growth and stabilisation in the eastern Kaapvaal craton. Terra Nov. 21, 219–228
Seward TM (1989) The hydrothermal chemistry of gold and its implication for ore formation: boiling and conductive cooling as examples. Econ Geol Monogr 6:398–404
Smith AJB, Beukes NJ, Gutzmer J (2013) The composition and depositional environments of Mesoarchaean iron formations of the West Rand Group of the Witwatersrand Supergroup, South Africa. Econ Geol 108:111–134
Southam G, Lengke MF, Fairbrother L, Reith F (2009) The biogeochemistry of gold. Elements 5:303–307
Sumner DY, Beukes NJ (2006) Sequence stratigraphic development of the Neoarchean Transvaal carbonate platform, Kaapvaal Craton, South Africa. S Afr J Geol 109:11–22
Taylor SR, McLennan SM (1985) The continental crust: its composition and evolution. Blackwell, London, 312 p
Thunell RC, Varela R, Llano M, Collister J, Muller-Karger F, Bohrer R (2000) Organic carbon fluxes, degradation, and accumulation in an anoxic basin: sediment trap results from the Cariaco Basin. ALSO 45:300–308
Tomkins AG (2013) A biogeochemical influence on the secular distribution of oro-genic gold. Econ Geol 108:193–197
Toulkeridis T, Clauer N, Kröner A, Todt W (2015) A mineralogical, chemical and isotopic investigation of shales from the Barberton Greenstone Belt, South Africa, to constrain source materials and post-deposition evolution. S Afr J Geol 118:389–410
Trinh LTT, Kjøniksen AL, Zhu K, Knudsen KD, Volden S, Glomm WR, Nystom B (2009) Slow salt-induced aggregation of citrate-covered silver particles in aqueous solutions of cellulose derivatives. Colloid Polym Sci 287:1391–1404
Tucker RF, Viljoen RP, Viljoen MJ (2016) A review of the Witwatersrand Basin - the world's greatest goldfield. Episodes 39:105–133
Tzoupanos ND, Zouboulis AI (2008) Coagulation-flocculation processes in water/wastewater treatment: the application of new generation of chemical reagents. 6th IASME/WSEAS International conference on heat transfer, thermal engineering and environment (hte'08) Rhodes, Greece, August 20–22
van Achterbergh E, Ryan CG, Jackson SE, Griffin W (2001) Data reduction software for LA–ICP–MS. In: Sylvester P (ed) Laser ablation ICPMS in the earth sciences, Mineralogical Association of Canada, Short Course Series, vol 29, pp 239–243
Wadnerkar D, Pareek VK, Utikar RP (2015) CFD modelling of flow and solids distribution in carbon–in–leach tanks. Metals 5:1997–2020
Wallmach T, Meyer FM (1990) A petrogenetic grid for metamorphosed aluminous Witwatersrand shales. S Afr J Geol 93:93–102
Watanabe Y, Naraoka H, Wronkiewicz DJ, Condie KC, Ohmoto H (1997) Carbon, nitrogen, and sulfur geochemistry of Archaean and Proterozoic shales from the Kaapvaal Craton, South Africa. Geochim Cosmochim Acta 61:3441–3459
Weitz DA, Huang JS, Lin MY, Sung J (1984) Dynamics of diffusion-limited kinetic aggregation. Phys Rev Lett 53:1657–1660
Wronkiewicz DJ, Condie KC (1987) Archaean shales from the Witwatersrand Supergroup, South Africa: source area weathering and provenance. Geochim Cosmochim Acta 51:2401–2416
Xue Y, Campbell I, Ireland T, Holden P, Armstrong R (2013) No mass-independent sulfur isotope fractionation in auriferous fluids supports a magmatic origin for Archean gold deposits. Geology 41:791–794
Yazici B, Yolacan S (2005) A comparison of various tests of normality. J Stat Comput Simul 77:175–183
Zeh A, Gerdes A, Barton JM Jr (2009) Archean accretion and crustal evolution of the Kalahari craton–the zircon age and Hf isotope record of granitic rocks from Barberton/Swaziland to the Francistown Arca. J Petrol 50:933–966
Zeh A, Ovtcharova M, Al W, Schaltegger U (2015) The bushveld complex was emplaced and cooled in less than one million years – results of zirconology, and geotectonic implications. Earth Planet Sci Lett 418:103–114
Acknowledgments
This work was supported by the National Research Foundation (NRF) of South Africa to G. Nwaila (Grant UID: 88323). We thank Sibanye Gold Limited for providing the Witwatersrand Supergroup samples, as well as Helene Brätz and Ulrich Schüßler for assistance with the ICP-MS and XRF analyses, respectively. Chris Heubeck and Axel Hofmann are acknowledged for supplying some of the Barberton Supergroup samples. We thank Ross Large and Christopher Lawley for their constructive reviews of the original manuscript and Maarten Broekmans for editorial handling.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial handling: D. Paktunc
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Fig. S1
Negative correlation between Au content and sediment provenance indicator elemental ratios (a - Zr/Ni, b – Th/Sc) for the Booysens Formation shales, indicating preferred derivation of gold from mafic source rocks. (PNG 4.62 MB)
Fig. S2
Index of chemical variability (ICV) versus chemical index of weathering (CIW) for marine shales of the Kaapvaal Craton. Notes: ICV = (CaO + K2O + Na2O + Fe2O3 (T) + MgO + MnO + TiO2)/Al2O3 and CIW = Al2O3/ (Al2O3 + Na2O + CaO*) × 100). Values for Archaean rain water pH are from Krupp et al. 1994, values for Archaean river water pH are from Frimmel 2005, and values from Archean seawater pH are from Halevy and Bachan 2017.(PNG 4.58 MB)
Supplementary data S1
Whole–rock major (wt%) and trace element (ppm) concentrations including gold and platinum group elements for marine shales of the Barberton, Witwatersrand and Transvaal supergroups. (XLSX 107 kb)
Rights and permissions
About this article
Cite this article
Nwaila, G.T., Frimmel, H.E. Highly siderophile elements in Archaean and Palaeoproterozoic marine shales of the Kaapvaal Craton, South Africa. Miner Petrol 113, 307–327 (2019). https://doi.org/10.1007/s00710-018-0650-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00710-018-0650-3