Abstract
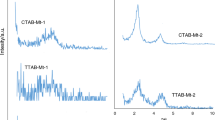
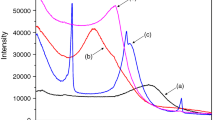
In this work, a novel organo-clays, zwitterionic surfactant modified montmorillonites (ZSMMs) were synthesized by using sulphobetaine and montmorillonites. The structures of ZSMMs were characterized by X ray diffraction (XRD) methods; the surfactant loading levels were measured by Total organic carbon (TOC) analysis, and their sorptive characteristics toward p-nitrophenol and nitrobenzene were investigated. XRD and TOC measurements indicated that the amount of adsorbed surfactants and the basal spacing of the ZSMMs increase with alkyl chain length and surfactant concentration. Sorption experiments showed that the capacity of p-nitrophenol to sorb onto the ZSMMs is higher than that of nitrobenzene. Both capacities increase with surfactant loading level; However, sorption capacity decreases when the surfactant concentration is higher than 2.0 CEC. Under the same surfactant loading level, the sorption capacities of p-nitrophenol and nitrobenzene increase with alkyl chain length. Under this experimental condition, the longer alkyl chain leads to a higher sorption capacity for hydrophobic organic compounds. On the basis of the ability of p-nitrophenol and nitrobenzene to sorb onto the montmorillonites, we conclude that the contaminant sorption coefficients, normalized with organic carbon content, highly depend on surfactant loading levels.






Similar content being viewed by others
References
Boyd SA, Mortland MM, Chiou CT (1988) Sorption characteristics of organic compounds on hexadecyltrimethylammonium-smectite. Soil Sci Soc Am J 52:652–657
Chen BL, Zhu LZ, Zhu JX, Xing BS (2005) Configurations of the bentonite-sorbed myristylpyridinium cation and their influences on the uptake of organic compounds. Environ Sci Technol 39:6093–6100
Frost RL, Liu R, Martens WN, Yuan Y (2008) Synthesis, characterization of mono, di and tri alkyl surfactant intercalated Wyoming montmorillonite for the removal of phenol from aqueous systems. J Colloid Interface Sci 327:287–294
Frost RL, Zhou Q, He HP, Xi YF (2007) Changes in the surfaces of adsorbed para-nitrophenol on HDTMA organoclay- The XRD and TG study. J Colloid Interface Sci 307:50–55
Gennari M, Messina C, Abbate C, Baglieri A, Boursier C (2009) Solubility and adsorption behaviors of chlorpyriphos-methyl in the presence of surfactants. J Environ Sci Health B 44:235–240
Grandjean J (2001) Interaction of a zwitterionic surfactant with synthetic clays in aqueous suspensions: a multinuclear magnetic resonance study. J Colloid Interface Sci 239:27–32
Guegan R (2010) Intercalation of a Nonionic Surfactant (C10E3) Bilayer into a Na-Montmorillonite Clay. Langmuir 26:19175–19180
He HP, Zhou Q, Martens WN, Kloprogge TJ, Yuan P, Yunfei XF, Zhui JX, Frost RL (2006) Microstructure of HDTMA+-modified montmorillonite and its influence on sorption characteristics. Clay Clay Miner 54:689–696
He HP, Zhou Q, Zhu JX, Shen W, Frost RL, Yuan P (2008) Mechanism of p-nitrophenol adsorption from aqueous solution by HDTMA+-pillared montmorillonite - Implications for water purification. J Hazard Mater 154:1025–1032
Khaodhiar S, Changchaivong S (2009) Adsorption of naphthalene and phenanthrene on dodecylpyridinium-modified bentonite. Appl Clay Sci 43:317–321
Lagaly G (1981) Characterization of clays by organic-compounds. Clay Miner 16:1–21
Li H, Zhang WH, Ding YJ, Boyd SA, Teppen BJ (2010) Sorption and desorption of carbamazepine from water by smectite clays. Chemosphere 81:954–960
Liao CJ, Chen CP, Wang MK, Chiang PN, Pai CW (2006) Sorption of chlorophenoxy propionic acids by organoclay complexes. Environ Toxicol 21:71–79
McLauchlin AR, Thomas NL (2008) Preparation and characterization of organoclays based on an amphoteric surfactant. J Colloid Interface Sci 321:39–43
McLauchlin AR, Thomas NL (2009) Preparation and thermal characterisation of poly(lactic acid) nanocomposites prepared from organoclays based on an amphoteric surfactant. Polym Degrad Stab 94:868–872
Meneghetti P, Qutubuddin S (2004) Synthesis of poly(methyl methaerylate) nanocomposites via emulsion polymerization using a zwitterionic surfactant. Langmuir 20:3424–3430
Qi LY, Fang Y, Wang ZY, Ma N, Jiang LY, Wang YY (2008) Synthesis and physicochemical investigation of long alkylchain betaine zwitterionic surfactant. J Surfactant Deterg 11:55–59
Rodriguez-Cruz MS, Sanchez-Martin MJ, Sanchez-Camazano M (2005) A comparative study of adsorption of an anionic and a non-ionic surfactant by soils based on physicochemical and mineralogical properties of soils. Chemosphere 61:56–64
Sanchez-Martin MJ, Dorado MC, del Hoyo C, Rodriguez-Cruz MS (2008) Influence of clay mineral structure and surfactant nature on the adsorption capacity of surfactants by clays. J Hazard Mater 150:115–123
Sheng GY, Wang XR, Wu SH, Boyd SA (1998) Enhanced sorption of organic contaminants by smectitic soils modified with a cationic surfactant. J Environ Qual 27:806–814
Sheng GY, Xu SH, Boyd SA (1996) Cosorption of organic contaminants from water by hexadecyltrimethylammonium-exchanged clays. Water Res 30:1483–1489
Wang T, Zhu JX, Zhu RL, Ge F, Yuan P, He HP (2010) Enhancing the sorption capacity of CTMA-bentonite by simultaneous intercalation of cationic polyacrylamide. J Hazard Mater 178:1078–1084
Yamaguchi Y, Hoffmann H (1997) Interaction between saponite and cationic, zwitterionic and nonionic surfactants. Colloid Surf A 121:67–80
Yang K, Zhu LZ, Xing BS (2007) Sorption of sodium dodecylbenzene sulfonate by montmorillonite. Environ Pollut 145:571–576
Zampori L, Stampino PG, Dotelli G (2009) Adsorption of nitrobenzene and orthochlorophenol on dimethyl ditallowyl montmorillonite: A microstructural and thermodynamic study. Appl Clay Sci 42:605–610
Zhou Q, He HP, Frost RL, Xi YF (2007) Adsorption of p-nitrophenol on mono-, di-, and trialkyl surfactant-intercalated organoclays: A comparative study. J Phys Chem C 111:7487–7493
Zhu DQ, Qu XL, Liu P (2008a) Enhanced sorption of polycyclic aromatic hydrocarbons to tetra-alkyl ammonium modified smectites via cation-pi interactions. Environ Sci Technol 42:1109–1116
Zhu JX, Qing YH, Wang T, Zhu RL, Wei JM, Tao Q, Yuan P, He HP (2011) Preparation and characterization of zwitterionic surfactant-modified montmorillonites. J Colloid Interface Sci 360:386–392
Zhu JX, Zhu LZ, Zhu RL, Chen BL (2008b) Microstructure of organo-bentonites in water and the effect of steric hindrance on the uptake of organic compounds. Clay Clay Miner 56:144–154
Zhu LZ, Chen BL (2000) Sorption behavior of p-nitrophenol on the interface between anion-cation organobentonite and water. Environ Sci Technol 34:2997–3002
Zhu LZ, Ma JF (2007) Removal of phenols from water accompanied with synthesis of organobentonite in one-step process. Chemosphere 68:1883–1888
Zhu LZ, Ruan XX, Chen BL, Zhu RL (2008c) Efficient removal and mechanisms of water soluble aromatic contaminants by a reduced-charge bentonite modified with benzyltrimethylammonium cation. Chemosphere 70:1987–1994
Acknowledgments
This work was financially supported by the grant of the Knowledge Innovation Program of the Chinese Academy of Sciences (KZCX2-EW-QN101), and “Strategic Priority Research Program” of the Chinese Academy of Sciences (Grant No. XDB05050200), National Natural Science Foundation of China (21177104, 41272060, U0933003).
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial handling: F. Dong
Rights and permissions
About this article
Cite this article
Zhu, J., Qing, Y., Ma, L. et al. The structure of montmorillonites modified with zwitterionic surfactants and their sorption ability. Miner Petrol 109, 349–355 (2015). https://doi.org/10.1007/s00710-014-0339-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00710-014-0339-1