Abstract
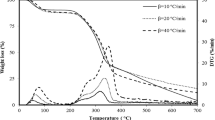
The thermal decompositions of Ca-bentonites (CaB) from Santai, Shichuan Province, China, over the temperature range of 30–1,100 °C were investigated by simultaneous thermal analyzer. Non-isothermal kinetic analysis was employed to study the thermal decomposition mechanism by using Netzsch Thermokinetics software. Flynn-Wall-Ozawa and Friedman isoconversional methods were used to calculate the activation energy and analyze the reaction steps. The probable mechanism and the corresponding kinetic parameters were determined by multivariate non-linear regression program. The results show that the thermal decomposition process of CaB over the temperature range of 30–800 °C is a kind of six-step, competitive reaction (F 1 D 3 F n C 1E F n F n model). The dehydration reaction is controlled by two consecutive mechanisms, nucleation and growth, followed by a diffusion-controlled reaction (F 1 D 3 model), the first step: E = 61.68 kJ mol−1, logA = 6.75 s−1; the second step: E = 50.73 kJ mol−1, logA = 3.11 s−1. The dehydroxylation reaction is controlled by three-step competitive mechanisms, an autocatalytically activated, initial reaction followed by n-order competitive reaction (C 1E F n F n model), the first step: E = 124.74 kJ mol−1, logA = 5.67 s−1; the second step: E = 245.29 kJ mol−1, logA = 11.69 s−1; the third step : E = 261.73 kJ mol−1, logA = 11.23 s−1. A combination reaction of the dehydration and dehydroxylation is observed, and controlled by one n-order reaction (F n model), E = 8.99 kJ mol−1, logA = −1.91 s−1.








Similar content being viewed by others
References
Avarmi M (1940) Kinetics of phase change II transformation relation for random distribution of nuclei. J Chem Phys 8:212–224
Bala P, Samantaray BK, Srivastava SK (2000) Dehydration transformation in Ca-montmorillonite. Bull Mater Sci 23:61–67
Bayram H, Önal M, Yılmaz H (2010) Thermal analysis of a white calcium bentonite. J Therm Anal Calorim 101:873–879
Bray HJ, Redfern SAT (1999) Kinetics of dehydration of Ca-montmorillonite. Phys Chem Miner 26:591–600
Bray HJ, Redfern SAT, Simon MC (1998) The kinetics of dehydration in Ca-montmorillonite: an in situ X-ray diffraction study. Mineral Mag 62:647–656
Budrugeac P, Segal E (2001) Some methodological problems concerning nonisothermal kinetic analysis of heterogeneous solid–gas reactions. Int J Chem Kinet 33:564–573
Ferrage E, Kirk CA, Cressey G, Cuadros J (2007) Dehydration of Ca-montmorillonite at the crystal scale. Part 2. Mechanisms and kinetics. Am Mineral 92:1007–1017
Flynn JH (1991) A general differential technique for the determination of parameters for d(α)/dt = f(α)A exp(−E/RT). J Therm Anal Calorim 37:293–305
Frost RL, Ruan H, Kloprogge JT, Gates WP (2000) Dehydration and dehydroxylation of nontronites and ferruginous smectite. Thermochim Acta 346:63–72
Girgis BS, Felix NS (1993) Kinetic dehydroxylation of nontronite, estimated from isothermal and nonisothermal thermogravimetry. J Therm Anal Calorim 32:1867–1876
Girgis BS, El-Barawy KA, Felix NS (1987) Dehydration kinetics of some smectites: a thermogravimetric study. Thermochim Acta 111:9–19
Grimm RE (1978) Gu¨ven N (1978) Bentonites geology, mineralogy, properties, and uses. Elsevier, Amsterdam
Guindy NM, El-Akkad TM, Flex NS, Nashed S (1985) Thermal dehydration of mono- and di-valent montmorillonite cationic derivates. Thermochim Acta 88:369–378
Horvath, Galikova L (1979) Mechanism of the H20(g) release during a dehydroxylation of montmorillonite. Chem Zvest 33:604–611
Killingley JS, Day SJ (1990) Dehydroxylation kinetics of kaolinite and montmorillonite from Queensland Tertiary oil shale deposits. Fuel 69:1145–1149
Koster van Groos AF, Guggenheim S (1986) Dehydration of K- exchanged montmorillonite at elevated temperatures and pressures. Clay Clay Miner 34:281–286
Koster van Groos AF, Guggenheim S (1989) Dehydroxylation of Ca- and Mg- exchanged montmorillonite. Am Mineral 74:627–636
Koster van Gross AF, Guggenheim S (1984) The effect of pressure on the dehydration reaction of interlayer water in Na-montmorillonite (Swy -1). Am Mineral 69:872–879
Laureiro Y, Jerez A, Rouquerol F, Rouquerol J (1996) Dehydration kinetics of Wyoming montmorillonite studied by controlled transformation rate thermal analysis. Thermochim Acta 278:165–173
Levy JH, Hurst HJ (1993) Kinetics of dehydroxylation, in nitrogen and water vapour, of kaolinite and smectite from Australian Tertiary oil shales. Fuel 72:873–877
Malhotra VM, Ogloza AA (1989) FTIR spectra of hydroxyls and dehydroxylation kinetics mechanism in montmorillonite. Phys Chem Miner 16:386–393
Noyan H, Önal M, Sarıkaya Y (2008) A model developed for acid dissolution thermodynamics of a Turkish bentonite. J Therm Anal Calorim 94:591–596
Önal M, Sarikaya Y (2007) Thermal behavior of a bentonite. J Therm Anal Calorim 90:167–172
Opfermann J, Blumm J (1998) Simulation of the sintering behavior of a ceramic green body using advanced thermokinetic analysis. Thermochim Acta 318:213–220
Opfermann J, Kaisersberger E, Flammersheim HJ (2002) Model-free analysis of thermoanalytical data- advantages and limitations. Thermochim Acta 391:119–127
Ozawa T (2000) Kinetic analysis by repeated temperature scanning. Part 1: theory and methods. Thermochim Acta 356:173–180
Poinsignon C, Yvon J, Mercier R (1982) Dehydration energy of the exchangeable cations in montmorillonite. A DTA study. Isr J Chem 22:253–255
Souza CEC, Nascimento RSV (2008) Adsorption behavior of cationic polymers on bentonite. J Therm Anal Calorim 94:579–583
Tan Ö, Yılmaz L, Zaimoğlu S et al (2004) Variation of some engineering properties of clays with heat treatment. Mater Lett 58:1176–1179
Taylor HFW (1962) Homogeneous and inhomogeneous mechanisms in the dehydroxylation of minerals. Clay Miner Bul 5:45–49
Varma RS (2002) Clay and clay-supported reagents in organic synthesis. Tetrahedron 58:1235–1255
Voga GP, Coelho MG et al (2000) Experimental and theoretical studies of the thermal behavior of titanium dioxide-SnO2 based composites. J Phys Chem A 115:2719–2726
Wang L, Zhang M, Redfern SAT, Zhang ZY (2002) Dehydroxylation and transformations of the 2:1 phyllosilicate pyrophyllite at elevated temperatures: an infrared spectroscopic study. Clay Clay Miner 50:272–283
Wang L, Zhang M, Redfern SAT (2003) Infrared spectroscopic study of CO2 incorporation into pyrophyllite [Al2Si4O10(OH)2] during dehydroxylation. Clay Clay Miner 51:439–444
Yılmaz MS, Kalpaklı Y, Pişkin S (2013) Thermal behavior and dehydroxylation kinetics of naturally occurring sepiolite and bentonite. J Therm Anal Calorim. doi:10.1007/s10973-013-3152-x
Zabat M, Van DH (2000) Evaluation of the energy barrier for dehydration of homoionic (Li, Na, Cs, Mg, Ca, Ba, Alx(OH)y z+ and La) montmorillonite by a differentiation method. Clay Miner 35:357–363
Acknowledgments
This work was supported by The National Land and Resources Public Welfare Scientific Research Project of China (201011005-5), National Natural Science Foundation of China (50974025), Specialized Research Fund for the Doctoral Program of Higher Education of China (20095122110015), Key Project of National Natural Science Foundation of China (41030426), and Scientific Research Foundation of the Education Ministry for Returned Chinese Scholars, China (2010-32).
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial handling: F. Dong
Rights and permissions
About this article
Cite this article
Zhang, Xh., He, C., Wang, L. et al. Non-isothermal kinetic analysis of thermal decomposition of the Ca-bentonite from Santai, China. Miner Petrol 109, 319–327 (2015). https://doi.org/10.1007/s00710-014-0331-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00710-014-0331-9