Abstract
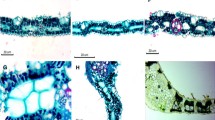
Phytomelanin is a brown to black pigment found in plant tissues, mainly in Asparagales and Asteraceae species. However, few studies deal with the processes of its synthesis, and there are still many questions to be answered regarding the organelles involved in this process and their functions, especially in vegetative organs. In a previous study with stems and leaves of 77 Vernonieae (Asteraceae) species, phytomelanin was demonstrated to always be associated with sclereids, which suggests the involvement of these cells in the pigment synthesis. Thus, we selected another species of tribe Vernonieae, Piptocarpha axillaris (Less.) Baker, which produces abundant phytomelanin secretion in stem tissues, to investigate which cells and organelles are involved in the synthesis and release of this pigment, as well as its distribution in the tissues. To achieve this goal, stems in different developmental phases were analyzed under light and transmission electron microscopy. Anatomical analysis showed that the polymerization of phytomelanin in P. axillaris starts at the second stem node, in the pith region, and occurs simultaneously with sclereid differentiation. The plastids of cells that will differentiate into sclereids actively participate in the phenolic material synthesis, following the “tannosome” and the “pearl necklace” models, giving rise to the main precursor of phytomelanin, which is then polymerized in the intercellular spaces during the sclerification process of sclereids. In stems with an established secondary structure, the pigment can be observed more frequently in the cortex, pericycle, primary phloem, secondary phloem, and pith.









Similar content being viewed by others
References
Asad M, Brahim M, Ziegler-Devin I, Boussetta N, Brosse N (2017) Chemical characterization of non-saccharidic and saccharidic components of rapeseed hulls and sunflower shells. Bioresources 12:3143–3153
Baldwin BG (2009) Heliantheae alliance. In: Funk VA, Susanna A, Stuessy T, Bayer R (eds) Systematics, evolution and biogeography of the Compositae. IAPT, Vienna, pp 689–711
Beckman CH (2000) Phenolic-storing cells: keys to programmed cell death and periderm formation in wilt disease resistance and in general defence responses in plants? Physiol Mol Plant P 57:101–110
Bremer K (1995) Asteraceae: cladistics and classification. Timber Press, Portland, pp176-178
Brillouet JM, Romieu C, Schoefs B, Solymosi K, Cheynier V, Fulcrand H, Verdeil JL, Conéjéro G (2013) The tannosome is an organelle forming condensed tannins in the chlorophyllous organs of Tracheophyta. Ann Bot 112:1003–1014
Brillouet JM (2014) Plasticity of the tannosome ontogenesis in the Tracheophyta. J Plant Sci 2:317–323
Brillouet JM (2015) On the role of chloroplasts in the polymerization of tannins in Tracheophyta: a monograph. Am J Plant Sci 6:1401–1409
Bukatsch F (1972) Bemerkungen zur Doppelfärbung Astrablau-Safranin. Mikrokosmos 6:255
Carlson EC, Knowles PF, Dillb JE (1972) Sunflower varietal resistance to sunflower moth larvae. Calif Agr 26:11–13
De-Paula OC, Marzinek J, Oliveira DMT, Machado SR (2013) The role of fibres and the hypodermis in Compositae melanin secretion. Micron 44:312–316
Driouich A, Faye L, Staehelin A (1993) The plant Golgi apparatus: a factory for complex polysaccharides and glycoproteins. Trends Biochem Sci 18:210–214
Fritz E, Saukel J (2011) Secretory structures of subterranean organs of some species of the Cardueae and their diagnostic value. Acta Biol Crac Ser Bot 53:62–72
Heubl GR, Bauer R, Wagner H (1988) Morphologische und anatomishe studien on Echinacea purpurea, E. angustifolia, E. pallida und Parthenium integrifolium. Sci Pharm 56:145–160
Huber H (1969) Die Samenmerkmale und Verwandtschaftsverhältnisse der Liliiflorae. Mitt Bot Staatssamml 8:219–538
Hutzler P, Fischbach R, Heller W, Jungblut TP, Reuber S, Schmitz R, Veit M, Weissenbo G, Schnitzler JP (1998) Tissue localization of phenolic compounds in plants by confocal laser scanning microscopy. J Exp Bot 49:953–965
Jeffrey C (2009) Evolution of Compositae flowers. In: Funk VA, Susanna A, Stuessy TF, Bayer RJ (eds) Systematics. Evolution and Biogeography of Compositae. IAPT, Vienna, pp 131–138
Johansen D (1940) Plant microtechnique. McGraw-Hill Book Co, Inc, New York, 523p
Keeley SC, Robinson H (2009) Vernonieae. In: Funk VA, Susana A, Stuessy TF, Bayer RJ (eds) Systematics, evolution, and biogeography of Compositae. IAPT, Viena, pp 439–470
Klabunde T, Eicken C, Sacchettini JC, Krebs B (1998) Crystal structure of a plant catechol oxidase containing a dicopper center. Nat Struct Mol Biol 5:1084–1090
Knowles PE (1978) Morphology and anatomy. In: Carter JF (ed) Sunflower Science and Technology. ASA, CSSA, SSSA, Inc, Madison, pp 55–87
Lusa MG, Loeuille BFP, Appezzato-da-Glória B (2018) First record of phytomelanin in aerial vegetative organs and its evolutionary implications in Lychnophorinae (Vernonieae: Asteraceae). Perspect Plant Ecol Syst 33:18–33
Mistríková I, Vaverková S (2007) Morphology and anatomy of Echinacea purpurea, E. angustifolia, E. pallida and Parthenium integrifolium. Biologia 62:2–5
Nikolaus RA, Piatelli M, Fattorusso E (1964) The structure of melanins and melanogenesis: IV. On some natural melanins. Tetrahedron 20:1163–1172
O’Brien TP (1976) Observations on the fine structure of the oat coleoptile I. The epidermal cells of the extreme apex. Protoplasma 63:385–416
Özdemir Ö, Keleş Y (2018) Extraction, purification, antioxidant properties and stability conditions of phytomelanin pigment on the sunflower seeds. Int J Second Metab 5:140–148
Pandey AK, Lee WW, Sack FD, Stuessy TF (1989) Development of the phytomelanin layer in fruits of Ageratum conyzoides (Compositae). Am J Bot 75:739–746
Pandey AK (1998) Development of phytomelanin in fruits of Tagetes patula (Asteraceae). J Indian Bot Soc77:35–38
Pandey AK, Dhakal MR (2001) Phytomelanin in Compositae. Curr Sci 80:933–940
Pandey AK, Stuessy TF, Mathur RR (2014) Phytomelanin and systematics of the Heliantheae alliance (Compositae). Plant Divers Evol 131:1–21
Panero JL (2007) Key to the tribes of the Heliantheae alliance. In: Kadereit JW, Jeffrey C (eds) The Families and Genera of Vascular Plants. Flowering Plants. Eudicots. Asterales. Springer, Berlin, pp 391–395
Park KIL, Ishikawa N, Morita Y, Choi JD, Hoshino A, Iida S (2007) A bHLH regulatory gene in the common morning glory, Ipomoea purpurea, controls anthocyanin biosynthesis in flowers, proanthocyanidin and phytomelanin pigmentation in seeds, and seed trichome formation. Plant J 49:641–654
Park KIL (2012) A bHLH protein partially controls proanthocyanidin and phytomelanin pigmentation in the seed coats of morning glory Ipomoea tricolor. Hort. Environ. Biotechnol 53:304–309
Pinard D, Mizrachi E (2018) Unsung and understudied: plastids involved in secondary growth. Curr Opin Plant Biol 42:30–36
Plonka PM, Grabacka M (2006) Melanin synthesis in microorganisms-biotechnological and medical aspects. Acta Biochim Pol 53:429–443
Qu S, Chapman N, Xia Z, Feng M, Feng S, Wang Z, Liu L (2017) Ultramicroscopy reveals a layer of multiply folded membranes around the tannin-accumulating vacuole in honeysuckle petal trichomes. Micron 99:1–8
Reynolds ES (1963) The use of lead citrate and high pH as an electron-opaque stain in electron microscopy. J Cell Biol 17:208–212
Rogers CE, Stafford RE, Kreitner GL (1982) Phytomelanin development and role in hybrid resistance to Homoeosoma electellum (Hulst.) larvae. In: Proceedings of the 10th International Sunflower Conference. Surfers Paradise, Australia, pp 138–141
Rogers CE, Kreitner GL (1983) Phytomelanin of sunflower achenes: a mechanism for pericarp resistance to abrasion by larvae of the sunflower moth (Lepidoptera: Pyralidae). Environ Entomol 2:1–9
Roshchina VV, Roshchina VD (1993) The elimination of substances in response to extreme factors. In: Roshchina VV, Roshchina VD (eds) The excretory Function of Higher Plants. Springer-Verlag, Berlin, pp 195–198
Sakai WS (1973) Simple method for differential staining of paraffin embedded plant material using toluidine blue O. Stain Technol 48:247–249
Sava VM, Yang SM, Hong MY, Yang PC, Huang GS (2001) Isolation and characterization of melanic pigments derived from tea and tea polyphenols. Food Chem 73:177–184
Shoeva OY, Mursalimov SR, Gracheva NV, Glagoleva AY, Börner A, Khlestkina EK (2020) Melanin formation in barley grain occurs within plastids of pericarp and husk cells. Sci Rep 10:1–9
Solano F (2014) Melanins: skin pigments and much more types, structural models, biological functions, and formation routes. New J Sci, pp:1–28
Thadeo M, Hampilos KE, Stevenson DW (2015) Anatomy of fleshy fruits in the monocots. Am J Bot 102:1757–1779
Upton R, Graff A, Jolliffe G, Laenger R, Williamson E (2011) American herbal pharmacopoeia: botanical pharmacognosy. Microscopic Characterization of Botanical Medicines. CRC Press, Boca Raton, p 810
Volet DP (2017) Estudo florístico e taxonômico do gênero Piptocarpha R. Br (Asteraceae: Vernonieae) no estado de São Paulo, Brasil. MSc Thesis, University of Campinas, Brazil, 123p.
Watson ML (1958) Staining of tissue sections for electron microscopy with heavy metals. J Cell Biol 4:475–478
Wittich PE, Graven P (1995) Histochemical study of the development of the phytomelan layer in the seed coat of Gasteria verrucosa (Mill.) H. Duval. Protoplasma 187:72–78
Wittich PE, Graven P (1998) Callose deposition and breakdown, followed by phytomelan, synthesis in the seed coat of Gasteria verrucosa (Mill.) H. Duval. Protoplasma 201:221–230
Zhao J, Pang Y, Dixon RA (2010) The mysteries of proanthocyanidin transport and polymerization. Plant Physiol 153:437–443
Acknowledgments
We are grateful to the Departamento de Botânica of the Universidade Federal de Santa Catarina - UFSC, especially to the Laboratório de Anatomia Vegetal (LAVEG) for the structure provided. The group wishes to thank the Laboratório Central de Microscopia Eletrônica (LCME-BIO-2020, UFSC) and the Laboratório Multiusuário de Estudos em Biologia (LAMEB, UFSC) for the use of its facilities. Thanks to the National Council for Scientific and Technological Development (CNPq) for the productivity grant (311721/2018-4) and the Coordination for the Improvement of Higher Education Personnel (CAPES) by the Master’s Degree fellowship granted to the first author (CAPES-DS from 01 March 2017 to 28 February 2019).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Handling Editor: Jaideep Mathur
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Coutinho, J.W., Rodrigues, A.C., Appezzato-da-Glória, B. et al. Plastid role in phytomelanin synthesis in Piptocarpha axillaris (Less.) Baker stems (Asteraceae, Vernonieae). Protoplasma 258, 963–977 (2021). https://doi.org/10.1007/s00709-021-01615-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-021-01615-3