Abstract
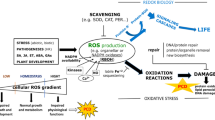
Absorption of excess excitation energy induces overproduction of singlet oxygen (1O2) in plants. The major sources of singlet oxygen production are chlorophyll and its intermediates located in the chloroplast. Over-accumulation of the chlorophyll biosynthetic intermediate protochlorophyllide by the exogenous application of 5-aminolevulinic acid (ALA), the precursor of tetrapyrrole, induced singlet oxygen production in the plastidic membranes. Over-expression of protochlorophyllide oxidoreductase C (PORC) in Arabidopsis thaliana resulted in efficient light-induced photo-transformation of protochlorophyllide to chlorophyllide that limited the accumulation of protochlorophyllide. Consequently, the 1O2 generation decreased in the PORC overexpressors (PORCx) and their cell death was minimal. Conversely, porC-2 over-accumulated protochlorophyllide in response to ALA treatment and generated higher amounts of 1O2 in light and had highest cell death as monitored by Evans blue staining. The protoplasts isolated from PORCx plants, when treated with ALA, generated minimal amounts of 1O2 as revealed by singlet oxygen sensor green (SOSG) fluorescence emission from chloroplasts. Conversely, the protoplasts of porC-2 mutants under identical conditions generated the maximum SOSG fluorescence in their chloroplasts and cytosol surrounding the chloroplasts most likely due to the leakage from the organelle. The membrane blebbing, a hallmark of programmed cell death, was clearly visible in WT and porC-2 protoplasts. Similarly, the nick end labelling (TUNEL) assay revealed nicks in the DNA. The TUNEL-positive nuclei after 30 min of light exposure were highest in porC-2 and lowest in PORCx protoplasts. The results demonstrate that higher amounts of singlet oxygen produced in the chloroplasts play an important role in programmed cell death.





Similar content being viewed by others
Abbreviations
- 1O2 :
-
Singlet oxygen
- ALA:
-
5-Aminolevulinic acid
- Chlide:
-
Chlorophyllide
- Chls:
-
Chlorophylls
- DAPI:
-
4′,6-diamidino-2-phenylindole
- DIC:
-
Differential interference contrast
- FDA:
-
Fluorescein diacetate
- FITC:
-
Fluorescein isothiocyanate
- Fv/Fm:
-
Ratio of variable fluorescence to maximal fluorescence
- PCD:
-
Programmed cell death
- Pchlide:
-
Protochlorophyllide
- PSI:
-
Photosystem I
- PSII:
-
Photosystem II
- POR:
-
Protochlorophyllide oxidoreductase
- ROS:
-
Reactive oxygen species
- SOSG:
-
Singlet oxygen sensor green
- TUNEL:
-
Terminal deoxynucleotide transferase dUTP nick end labelling
References
Ambastha V, Sopory SK, Tiwari BS, Tripathy BC (2017) Photo-modulation of programmed cell death in rice leaves triggered by salinity. Apoptosis 22:41–56
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Armstrong GA, Runge S, Frick G, Sperling U, Apel K (1995) Identification of NADPH:protochlorophyllide oxidoreductases a and B: a branched pathway for light-dependent chlorophyll biosynthesis in Arabidopsis thaliana. Plant Physiol 108:1505–1517
Asada K (1984) Chloroplasts:formation of active oxygen and its scavenging. Methods Enzymol 105:422–429
Bras M, Queenan B, Susin SA (2005) Programmed cell death via mitochondria: different modes of dying. Biochem 70:231–239
Casolo V, Petrussa E, Krajňáková J et al (2005) Involvement of the mitochondrial KATP+ channel in H2O2 - or NO-induced programmed death of soybean suspension cell cultures. J Exp Bot 56:997–1006
Chakraborty N, Tripathy BC (1992) Involvement of singlet oxygen in 5-aminolevulinic acid-induced photodynamic damage of cucumber (Cucumis sativus l.) chloroplasts. Plant Physiol 98:7–11
D’Alessandro S, Havaux M (2019) Sensing β-carotene oxidation in photosystem II to master plant stress tolerance. New Phytol. https://doi.org/10.1111/nph.15924
Danon A, Coll N, Apel K (2006) Cryptochrome-1-dependent execution of programmed cell death induced by singlet oxygen in Arabidopsis thaliana. PNAS 103:17036–17041
Foote CS (1991) Definition of type I and type I1. Photochem Photobiol 54:659
He R, Drury GE, Rotari VI, Gordon A, Willer M, Farzaneh T, Woltering EJ, Gallois P (2008) Metacaspase-8 modulates programmed cell death induced by ultraviolet light and H2O2 in Arabidopsis. J Biol Chem 283:774–783
Hukmani P, Tripathy BC (1992) Spectrofluorometric estimation of intermediates of chlorophyll biosynthesis: Protoporphyrin IX, mg-protoporphyrin, and protochlorophyllide. Anal Biochem 206:125–130
Jung J, Kim HS (1990) The chromophores as endogenous sensitizers involved in the photogeneration of singlet oxygen in spinach thylakoids. Photochem Photobiol 52:1003–1009
Kandoi D, Mohanty S, Govindjee TBC (2016) Towards efficient photosynthesis: overexpression of Zea mays phosphoenolpyruvate carboxylase in Arabidopsis thaliana. Photosynth Res 130:47–72
Kim C, Meskauskiene R, Apel K, Laloi C (2008) No single way to understand singlet oxygen signalling in plants. EMBO Rep 9:435–439
Krieger-Liszkay A, Trebst A (2006) Tocopherol is the scavenger of singlet oxygen produced by the triplet states of chlorophyll in the PSII reaction Centre. J Exp Bot 57:1677–1684
Laloi C, Havaux M (2015) Key players of singlet oxygen-induced cell death in plants. Front Plant Sci 6:1–9
Lee KP, Kim C, Landgraf F, Apel K (2007) EXECUTER1- and EXECUTER2-dependent transfer of stress-related signals from the plastid to the nucleus of Arabidopsis thaliana. Proc Natl Acad Sci U S A 104:10270–10275
Lin J, Wang Y, Wang G (2006) Salt stress-induced programmed cell death in tobacco protoplasts is mediated by reactive oxygen species and mitochondrial permeability transition pore status. J Plant Physiol 163:731–739
Masuda T, Fusada N, Oosawa N, Takamatsu K, Yamamoto YY, Ohto M, Nakamura K, Goto K, Shibata D, Shirano Y, Hayashi H, Kato T, Tabata S, Shimada H, Ohta H, Takamiya K (2003) Functional analysis of isoforms of NADPH:protochlorophyllide oxidoreductase (POR), PORB and PORC, in Arabidopsis thaliana. Plant Cell Physiol 44:963–974
McManus MJ, Murphy MP, Franklin JL (2014) Mitochondria-derived reactive oxygen species mediate caspase-dependent and -independent neuronal deaths. Mol Cell Neurosci 63:13–23
Meskauskiene R, Nater M, Goslings D et al (2001) FLU: a negative regulator of chlorophyll biosynthesis in Arabidopsis thaliana. Proc Natl Acad Sci 98:12826–12831
Mittler R (2017) ROS are good. Trends Plant Sci 22:11–19. https://doi.org/10.1016/j.tplants.2016.08.002
Mohapatra A, Tripathy BC (2002) Detection of protoporphyrin IX in envelope membranes of pea chloroplasts. Biochem Biophys Res Commun 299:751–754
Mohapatra A, Tripathy BC (2007) Differential distribution of chlorophyll biosynthetic intermediates in stroma, envelope and thylakoid membranes in Beta vulgaris. Photosynth Res 94:401–410
Oosawa N, Masuda T, Awai K, Fusada N, Shimada H, Ohta H, Takamiya K (2000) Identification and light-induced expression of a novel gene of NADPH-protochlorophyllide oxidoreductase isoform in Arabidopsis thaliana. FEBS Lett 474:133–136
op den Camp RGL (2003) Rapid induction of distinct stress responses after the release of singlet oxygen in Arabidopsis. Plant Cell 15:2320–2332
Pattanayak GK, Tripathy BC (2002) Catalytic function of a novel protein protochlorophyllide oxidoreductase C of Arabidopsis thaliana. Biochem Biophys Res Commun 291:921–924
Pattanayak GK, Tripathy BC (2011) Overexpression of Protochlorophyllide oxidoreductase C regulates oxidative stress in Arabidopsis. PLoS One 6. https://doi.org/10.1371/journal.pone.0026532
Ramel F, Birtic S, Cuine S et al (2012) Chemical quenching of singlet oxygen by carotenoids. Plant Physiol 158:1267–1278
Ramel F, Ksas B, Akkari E, Mialoundama AS, Monnet F, Krieger-Liszkay A, Ravanat JL, Mueller MJ, Bouvier F, Havaux M (2013a) Light-induced acclimation of the Arabidopsis chlorina1 mutant to singlet oxygen. Plant Cell 25:1445–1462
Ramel F, Ksas B, Havaux M (2013b) A decision maker between cell death and acclimation in the response of plants to singlet oxygen Jasmonate. Plant Signal Behav 8:e26655
Rebeiz CA, Zouhoor AM, Mayasich JM et al (1988) Photodynamic herbicides . Recent developments and molecular basis of selectivity. CRC Crit Rev Plant Sci 6:385–436
Reinbothe S, Reinbothe C, Lebedev N, Apel K (1996) PORA and PORB, protochlorophyllide-reducing enzymes of angiosperm chlorophyll biosynthesis. Plant Cell 8:763–769
Sekine S, Ichijo H (2015) Mitochondrial proteolysis: its emerging roles in stress responses. Biochim Biophys Acta, Gen Subj 1850:274–280
Shumbe L, Chevalier A, Legeret B, Taconnat L, Monnet F, Havaux M (2016) Singlet oxygen-induced cell death in Arabidopsis under high light stress is controlled by OXI1 kinase. Plant Physiol 170:1757–1771
Su Q, Armstrong G, Apel K (2001) POR C of Arabidopsis thaliana : a third light- and NADPH-dependent protochlorophyllide oxidoreductase that is differentially regulated by light. Plant Mol Biol 47:805–813
Tewari AK, Tripathy BC (1998) Temperature-stress-induced impairment of chlorophyll biosynthetic reactions in cucumber and wheat. Plant Physiol 117:851–858
Tiwari BS, Belenghi B, Levine A (2002) Oxidative stress increased respiration and generation of reactive oxygen species, resulting in ATP depletion, opening of mitochondrial permeability transition, and programmed cell death. Plant Physiol 128:1271–1281
Tripathy BC, Chakraborty N (1991) 5-Aminolevulinic acid induced photodynamic damage of the photosynthetic electron transport chain of cucumber (Cucumis sativus L.) cotyledons. Plant Physiol 96:761–767
Tripathy BC, Oelmüller R (2012) Reactive oxygen species generation and signaling in plants. Plant Signal Behav 7:1621–1633
Tripathy BC, Pattanayak G (2010) Singlet oxygen-induced oxidative stress in plants. In: Rebeiz CA, Benning C, Bohnert HJ et al (eds) The chloroplast: basics and applications. Springer Netherlands, Dordrecht, pp 397–412
Tripathy BC, Mohapatra A, Gupta I (2007) Impairment of the photosynthetic apparatus by oxidative stress induced by photosensitization reaction of protoporphyrin IX. Biochim Biophys Acta Bioenerg 1767:860–868
Vass I (2011) Role of charge recombination processes in photodamage and photoprotection of the photosystem II complex. Physiol Plant 142:6–16
Vass I (2012) Molecular mechanisms of photodamage in the photosystem II complex. Biochim Biophys Acta Bioenerg 1817:209–217
Vedalankar P, Tripathy BC (2019) Evolution of light-independent protochlorophyllide oxidoreductase. Protoplasma 256:293–312
Vijayaraghavareddy P, Adhinarayanreddy V, Vemanna RS et al (2017) Quantification of membrane damage/cell death using evan’s blue staining technique. Bio-protocol 7:e2519
Wang Y, Li Y, Xue H, Pritchard HW, Wang X (2015) Reactive oxygen species-provoked mitochondria-dependent cell death during ageing of elm (Ulmus pumila L.) seeds. Plant J 81:438–452
Waszczak C, Carmody M, Kangasjarvi J (2018) Reactive oxygen species in plant signaling. Annu Rev Plant Biol 69:209–236
Funding
This work was supported by a grant from the Department of Science and Technology, Government of India (EMR/2016/004976) and BSR Faculty Fellowship from the University Grants Commission to BCT. BST received financial support from DST/SERB (No EMR/2016/002732).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Jaideep Mathur
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ambastha, V., Chauhan, G., Tiwari, B.S. et al. Execution of programmed cell death by singlet oxygen generated inside the chloroplasts of Arabidopsis thaliana. Protoplasma 257, 841–851 (2020). https://doi.org/10.1007/s00709-019-01467-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-019-01467-y