Abstract
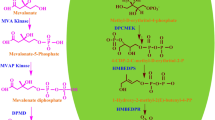
Camptothecin is a high-value anti-cancerous compound produced in many taxonomically unrelated species. Its biosynthesis involves a complex network of pathways and a diverse array of intermediates. Here, we report the functional characterization and regulation of secologanin synthase (NnCYP72A1), a cytochrome P450 involved in camptothecin biosynthesis from Nothapodytes nimmoniana. It comprises an open reading frame of 1566 bp in length. Heterologous expression in Saccharomyces cerevisiae and in vitro enzymatic assays using loganin as substrate confirmed the formation of secologanin. In planta transient overexpression analysis of NnCYP72A1 resulted in 4.21- and 2.73-fold increase in transcript levels of NnCYP72A1 on days 3 and 6 respectively. Phytochemical analysis of transformed tissues revealed ~ 1.13–1.43- and 2.02–2.86-fold increase in secologanin and CPT accumulation, respectively. Furthermore, promoter analysis of NnCYP72A1 resulted in the identification of several potential cis-regulatory elements corresponding to different stress-related components. Methyl jasmonate, salicylic acid, and wounding treatments resulted in considerable modulation of mRNA transcripts of NnCYP72A1 gene. Chemical analysis of elicitor-treated samples showed a significant increase in CPT content which was concordant with the mRNA transcript levels. Overall, the functional characterization and overexpression of NnCYP72A1 may plausibly enhance the pathway intermediates and serve as prognostic tool for enhancing CPT accumulation.







Similar content being viewed by others
References
Ali MB, Hahn EJ, Paek KY (2007) Methyl jasmonate and salicylic acid induced oxidative stress and accumulation of phenolics in Panax ginseng bioreactor root suspension cultures. Molecules. 12:607–621
Ali M, Abbasi BH, Ali GS (2015) Elicitation of antioxidant secondary metabolites with jasmonates and gibberellic acid in cell suspension cultures of Artemisia absinthium L. Plant Cell Tissue Organ Cult 120:1099–1106
Asada K, Salim V, Masada-Atsumi S, Edmunds E, Nagatoshi M, Terasaka K, Mizukami H, De Luca VA (2013) 7-deoxyloganetic acid glucosyltransferase contributes a key step in secologanin biosynthesis in Madagascar periwinkle. Plant Cell 25:4123–4134. https://doi.org/10.1105/tpc.113.115154
Ashkenazy H, Erez E, Martz E, Pupko T, Ben-Tal N (2010) ConSurf 2010: calculating evolutionary conservation in sequence and structure of proteins and nucleic acids. Nucleic Acids Res 38:529–533
Bhat WW, Dhar N, Razdan S, Rana S, Mehra R, Nargotra A, Dhar RS, Ashraf N, Vishwakarma R, Lattoo SK (2013) Molecular characterization of UGT94F2 and UGT86C4, two glycosyltransferases from Picrorhiza kurrooa: comparative structural insight and evaluation of substrate recognition. PLoS One 8(9):e73804. https://doi.org/10.1371/journal.pone.0073804
Biłas R, Szafran K, Hnatuszko-Konka K, Kononowicz AK (2016) Cis-regulatory elements used to control gene expression in plants. Plant Cell Tissue Organ Cult 127:269–287
Chen F, Li W, Jiang L, Pu X, Yang Y, Zhang G, Luo Y (2016) Functional characterization of a geraniol synthase-encoding gene from Camptotheca acuminata and its application in production of geraniol in Escherichia coli. J Ind Microbiol Biotechnol 43:1281–1292
Cheong Y, Chang HS, Gupta R, Wang X, Zhu T, Luan S (2002) Transcriptional profiling reveals novel interactions between wounding, pathogen, abiotic stress, and hormonal responses in Arabidopsis. Plant Physiol 129:661–677
Cui X, Wang YX, Liu ZW, Wang WL, Li H, Zhuang J (2018) Transcriptome-wide identification and expression profile analysis of the bHLH family genes in Camellia sinensis. Funct Integr Genomics 18:489–503. https://doi.org/10.1007/s10142-018-0608-x
de Bernonville TD, Foureau E, Parage C, Lanoue A, Clastre M, Londono MA, Oudin A, Houillé B, Papon N, Besseau S, Glévarec G (2015) Characterization of a second secologanin synthase isoform producing both secologanin and secoxyloganin allows enhanced de novo assembly of a Catharanthus roseus transcriptome. BMC Genomics 16:619. https://doi.org/10.1186/s12864-015-1678-y
De Geyter N, Gholami A, Goormachtig S, Goossens A (2012) Transcriptional machineries in jasmonate-elicited plant secondary metabolism. Trends Plant Sci 17:349–359
De Luca V, Salim V, Thamm A, Masada SA, Yu F (2014) Making iridoids/secoiridoids and monoterpenoid indole alkaloids: progress on pathway elucidation. Curr Opin Plant Biol 19:35–42. https://doi.org/10.1016/j.pbi.2014.03.006
Dhar N, Rana S, Razdan S, Bhat WW, Hussain A, Dhar RS, Vaishnavi S, Hamid A, Vishwakarma R, Lattoo SK (2014) Cloning and functional characterization of three branch point oxidosqualene cyclases from Withania somnifera (L.) dunal. J Biol Chem 289:17249–17267
Fulzele DP, Satdive RK (2005) Comparison of techniques for the extraction of the anti-cancer drug camptothecin from Nothapodytes foetida. J Chromatogr 1063:9–13. https://doi.org/10.1016/j.chroma.2004.11.020
Giddings LA, Liscombe DK, Hamilton JP, Childs KL, DellaPenna D, Buell CR, O’Connor SE (2011) A stereoselective hydroxylation step of alkaloid biosynthesis by a unique cytochrome P450 in Catharanthus roseus. J Biol Chem 286:16751–16757
Gietz RD, Schiestl RH (2007) High-efficiency yeast transformation using the LiAc/SS carrier DNA/PEG method. Nat Protoc 2:31–34
Góngora-Castillo E, Childs KL, Fedewa G, Hamilton JP, Liscombe DK, Magallanes-Lundback M, Mandadi KK, Nims E, Runguphan W, Vaillancourt B, Varbanova-Herde M (2012) Development of transcriptomic resources for interrogating the biosynthesis of monoterpene indole alkaloids in medicinal plant species. PLoS One 7:e52506. https://doi.org/10.1371/journal.pone.0052506
Guirimand G, Courdavault V, Lanoue A, Mahroug S, Guihur A, Blanc N, Giglioli-Guivarc’h N, St-Pierre B, Burlat V (2010) Strictosidine activation in Apocynaceae: towards a “nuclear time bomb”? BMC Plant Biol 10:182. https://doi.org/10.1186/1471-2229-10-182
Hernandez-Garcia CM, Finer JJ (2014) Identification and validation of promoters and cis-acting regulatory elements. Plant Sci 217:109–119
Höfer R, Dong L, André F, Ginglinger JF, Lugan R, Gavira C, Grec S, Lang G, Memelink J, Van Der Krol S, Bouwmeester H (2013) Geraniol hydroxylase and hydroxygeraniol oxidase activities of the CYP76 family of cytochrome P450 enzymes and potential for engineering the early steps of the (seco) iridoid pathway. Metab Eng 20:221–232
Höfer R, Boachon B, Renault H, Gavira C, Miesch L, Iglesias J, Ginglinger JF, Allouche L, Miesch M, Grec S, Larbat R (2014) Dual function of the cytochrome P450 CYP76 family from Arabidopsis thaliana in the metabolism of monoterpenols and phenylurea herbicides. Plant Physiol 166:1149–1161
Huang FC, Sung PH, Do YY, Huang PL (2012) Differential expression and functional characterization of the NADPH cytochrome P450 reductase genes from Nothapodytes foetida. Plant Sci 190:16–23
Huang Y, Tan H, Guo Z, Wu X, Zhang Q, Zhang L, Diao Y (2016) The biosynthesis and genetic engineering of bioactive indole alkaloids in plants. J Plant Biol 59:203–214
Irmler S, Schröder G, St-Pierre B, Crouch NP, Hotze M, Schmidt J, Strack D, Matern U, Schröder J (2000) Indole alkaloid biosynthesis in Catharanthus roseus: new enzyme activities and identification of cytochrome P450 CYP72A1 as secologanin synthase. Plant J 24:797–804
Isah T, Mujib A (2015) Camptothecin from Nothapodytes nimmoniana: review on biotechnology applications. Acta Physiol Plant 37. https://doi.org/10.1007/s11738-015-1854-3
Käll L, Krogh A, Sonnhammer EL (2007) Advantages of combined transmembrane topology and signal peptide prediction—the Phobius web server. Nucleic Acids Res 35:429–432
Kandel S, Sauveplane V, Compagnon V, Franke R, Millet Y, Schreiber L, Werck-Reichhart D, Pinot F (2007) Characterization of a methyl jasmonate and wounding responsive cytochrome P450 of Arabidopsis thaliana catalyzing dicarboxylic fatty acid formation in vitro. FEBS J 274:5116–5127
Kelley LA, Mezulis S, Yates CM, Wass MN, Sternberg MJ (2015) The Phyre2 web portal for protein modeling, prediction and analysis. Nat Protoc 10:845–858
Kim HJ, Chen F, Wang X, Rajapakse NC (2006) Effect of methyl jasmonate on secondary metabolites of sweet basil (Ocimum basilicum L.). J Agric Food Chem 54:2327–2332
Krithika R, Srivastava P, Rani B, Kolet SP, Chopade M, Soniya M, Thulasiram HV (2015) Characterization of 10-hydroxygeraniol dehydrogenase from Catharanthus roseus reveals cascaded enzymatic activity in iridoid biosynthesis. Sci Rep 5:8258. https://doi.org/10.1038/srep08258
Kumar A, Giridhar P (2015) Salicylic acid and methyljasmonate restore the transcription of caffeine biosynthetic N-methyltransferases from a transcription inhibition noticed during late endosperm maturation in coffee. Plant Gene 4:38–44
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874
Kumar SR, HB S, Nagegowda DA (2018) Terpene moiety enhancement by overexpression of geranyl (geranyl) diphosphate synthase and geraniol synthase elevates monomeric and dimeric monoterpene indole alkaloids in transgenic Catharanthus roseus. Front Plant Sci 9:942
Leonard E, Runguphan W, O’connor S, Prather KJ (2009) Opportunities in metabolic engineering to facilitate scalable alkaloid production. Nat Chem Biol 5:292–300
Li CY, Leopold AL, Sander GW, Shanks JV, Zhao L (2013) Gibson SI. The ORCA2 transcription factor plays a key role in regulation of the terpenoid indole alkaloid pathway. BMC Plant Biol 13:155. https://doi.org/10.1186/1471-2229-13-155
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods. 25:402–408
Lorence A, Nessler CL (2004) Camptothecin, over four decades of surprising findings. Phytochemistry 65:2735–2749
Magadum S, Banerjee U, Murugan P, Gangapur D, Ravikesavan R (2013) Gene duplication as a major force in evolution. J Genet 92:155–116
Miettinen K, Dong L, Navrot N, Schneider T, Burlat V, Pollier J, Woittiez L, Van Der Krol S, Lugan R, Ilc T, Verpoorte R (2014) The seco-iridoid pathway from Catharanthus roseus. Nat Commun 5:3606
Mizutani M (2012) Impacts of diversification of cytochrome P450 on plant metabolis. Biol Pharm Bull 35:824–832
Moerkercke AV, Steensma P, Schweizer F, Pollier J, Gariboldi I, Payne R, Bossche RV, Miettinen K, Espoz J, Purnama PC, Kellner F (2015) The bHLH transcription factor BIS1 controls the iridoid branch of the monoterpenoid indole alkaloid pathway in Catharanthus roseus. Proc Natl Acad Sci 112:8130–8135
Murata J, Roepke J, Gordon H, De Luca V (2008) The leaf epidermome of Catharanthus roseus reveals its biochemical specialization. Plant Cell 20:524–542
Omura T, Sato R (1964) The carbon monoxide-binding pigment of liver microsomes I. Evidence for its hemoprotein nature. J Biol Chem 239:2370–2378
Pan Q, Mustafa NR, Tang K, Choi YH, Verpoorte R (2016) Monoterpenoid indole alkaloids biosynthesis and its regulation in Catharanthus roseus: a literature review from genes to metabolites. Phytochem Rev 15:221–250
Pandith SA, Dhar N, Rana S, Bhat WW, Kushwaha M, Gupta AP, Shah MA, Vishwakarma R, Lattoo SK (2016) Characterization and functional promiscuity of two divergent paralogs of type III plant polyketide synthases from Rheum emodi Wall ex. Meissn. Plant Physiol. https://doi.org/10.1104/pp.16.00003
Pathania S, Bagler G, Ahuja PS (2016) Differential network analysis reveals evolutionary complexity in secondary metabolism of Rauvolfia serpentina over Catharanthus roseus. Front Plant Sci 7:1229
Peebles CA, Hughes EH, Shanks JV, San KY (2009) Transcriptional response of the terpenoid indole alkaloid pathway to the overexpression of ORCA3 along with jasmonic acid elicitation of Catharanthus roseus hairy roots over time. Metab Eng 11:76–86
Pommier Y (2009) DNA topoisomerase I inhibitors: chemistry, biology, and interfacial inhibition. Chem Rev 109:2894–2902
Pompon D, Louerat B, Bronine A, Urban P (1996) Yeast expression of animal and plant P450s in optimized redox environments. Methods Enzymol 272:51–64
Prall W, Hendy O, Thornton LE (2016) Utility of a phylogenetic perspective in structural analysis of CYP72A enzymes from flowering plants. PLoS One. https://doi.org/10.1371/journal.pone.0163024
Qu Y, Easson ML, Froese J, Simionescu R, Hudlicky T, De Luca V (2015a) Completion of the seven-step pathway from tabersonine to the anticancer drug precursor vindoline and its assembly in yeast. Proc Natl Acad Sci 112:6224–6229
Qu X, Pu X, Chen F, Yang Y, Yang L, Zhang G, Luo Y (2015b) Molecular cloning, heterologous expression, and functional characterization of an NADPH-cytochrome P450 reductase gene from Camptotheca acuminata, a camptothecin-producing plant. PLoS One 10. https://doi.org/10.1371/journal.pone.0135397
Ramesha BT, Amna T, Ravikanth G, Gunaga RP, Vasudeva R, Ganeshaiah KN, Uma Shaanker R, Khajuria RK, Puri SC, Qazi GN (2008) Prospecting for camptothecines from Nothapodytes nimmoniana in the Western Ghats, South India: identification of high-yielding sources of camptothecin and new families of camptothecines. J Chromatogr Sci 46(4):362–368. https://doi.org/10.1093/chromsci/46.4.362
Rather GA, Sharma A, Pandith SA, Kaul V, Nandi U, Misra P, Lattoo SK (2018) De novo transcriptome analyses reveals putative pathway genes involved in biosynthesis and regulation of camptothecin in Nothapodytes nimmoniana (Graham) Mabb. Plant Mol Biol 96:197–215
Sadre R, Magallanes-Lundback M, Pradhan S, Salim V, Mesberg A, Jones AD, DellaPenna D (2016) Metabolite diversity in alkaloid biosynthesis: a multi-lane (diastereomer) highway for camptothecin synthesis in Camptotheca acuminate. Plant Cell 28:1926–1944. https://doi.org/10.1105/tpc.16.00193
Salim V, Wiens B, Masada-Atsumi S, Yu F, De Luca V (2014) 7-deoxyloganetic acid synthase catalyzes a key 3 step oxidation to form 7-deoxyloganetic acid in Catharanthus roseus iridoid biosynthesis. Phytochemistry. 101:23–31
Savatin DV, Gramegna G, Modesti V, Cervone F (2014) Wounding in the plant tissue: the defense of a dangerous passage. Front Plant Sci 5:470. https://doi.org/10.3389/fpls.2014.00470
Shakya P, Marslin G, Siram K, Beerhues L, Franklin G (2019) Elicitation as a tool to improve the profiles of high-value secondary metabolites and pharmacological properties of Hypericum perforatum. J Pharm Pharmacol 71:70–82
Sharma A, Verma P, Mathur A, Mathur AK (2018) Overexpression of tryptophan decarboxylase and strictosidine synthase enhanced terpenoid indole alkaloid pathway activity and antineoplastic vinblastine biosynthesis in Catharanthus roseus. Protoplasma 255(5):1281–1294. https://doi.org/10.1007/s00709-018-1233-1
Shukla AK, Shasany AK, Gupta MM, Khanuja SP (2006) Transcriptome analysis in Catharanthus roseus leaves and roots for comparative terpenoid indole alkaloid profiles. J Exp Bot 57:3921–3932
Singh RS, Kesari R, Kumar U, Jha VK, Kumar A, Kumar T, Pal AK, Singh PK (2018) Candidate genes of flavonoid biosynthesis in Selaginella bryopteris (L.) Baker identified by RNA-Seq. Funct Integr Genomics 18:505–517. https://doi.org/10.1007/s10142-018-0603-2
Sun Y, Luo H, Li Y, Sun C, Song J, Niu Y, Zhu Y, Dong L, Lv A, Tramontano E, Chen S (2011) Pyrosequencing of the Camptotheca acuminata transcriptome reveals putative genes involved in camptothecin biosynthesis and transport. BMC Genomics 12. https://doi.org/10.1186/1471-2164-12-533
Yamazaki M, Mochida K, Asano T, Nakabayashi R, Chiba M, Udomson N, Yamazaki Y, Goodenowe DB, Sankawa U, Yoshida T, Toyoda A (2013) Coupling deep transcriptome analysis with untargeted metabolic profiling in Ophiorrhiza pumila to further the understanding of the biosynthesis of the anti-cancer alkaloid camptothecin and anthraquinones. Plant Cell Physiol 54:686–696
Yang L, Ding G, Lin H, Cheng H, Kong Y, Wei Y, Fang X, Liu R, Wang L, Chen X, Yang C (2013) Transcriptome analysis of medicinal plant Salvia miltiorrhiza and identification of genes related to tanshinone biosynthesis. PLoS One 8:e80464. https://doi.org/10.1371/journal.pone.0080464
Zhao L, Sander GW, Shanks JV (2013) Perspectives of the metabolic engineering of terpenoid indole alkaloids in Catharanthus roseus hairy roots. Adv Biochem Eng Biotechnol 134:23–54
Acknowledgments
Authors are thankful to Dr. Utpal Nandi at CSIR-IIIM, Jammu for facilitating LC-MS/MS analyses. We are also thankful to Nicolas Navrot, University de Strasbourg, France, for providing pYeDP60 vector and Wat11 strain. GAR is thankful to UGC for providing Senior Research Fellowship. AS thankfully acknowledges the DST-INSPIRE Senior Research Fellowship. This manuscript represents Institutional Communication No. CSIR/IIIM/IPR/0064.
Funding
This work was supported by a financial grant from Council of Scientific and Industrial Research (CSIR)-Indian Institute of Integrative Medicine under Major Lab Project MLP-3012 (WP 7).
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: SKL. Performed the experiments: GAR, AS, AK. Analyzed the data: GAR, SKL, VK, PM. Contributed reagents/materials/analysis tools: SKL. Original draft of the manuscript was prepared by GAR. SKL, PM, and VK improved the content and edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Handling Editor: Peter Nick
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary file1
Primers used for amplification and expression of NnCYP72A1. (DOCX 17 kb)
Supplementary file 2
Conserved residue prediction for NnCYP72A1 and multiple sequence alignment. (DOCX 324 kb)
Supplementary file 3
Enzyme kinetics of NnCYP72A1. (XLSX 537 kb)
Supplementary file 4
Nucleotide sequences of NnCYP72A1 gene promoter. (DOCX 1111 kb)
Supplementary file 5
Chemical profiles of tissue extracts for detection and quantification of camptothecin. (XLSX 336 kb)
Rights and permissions
About this article
Cite this article
Rather, G.A., Sharma, A., Misra, P. et al. Molecular characterization and overexpression analyses of secologanin synthase to understand the regulation of camptothecin biosynthesis in Nothapodytes nimmoniana (Graham.) Mabb.. Protoplasma 257, 391–405 (2020). https://doi.org/10.1007/s00709-019-01440-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-019-01440-9