Abstract
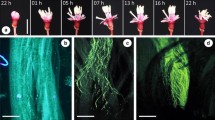
We analyzed the gynoecium morphology and anatomy of Tricomaria usillo in young and mature flowers from diverse populations in order to analyze the differentiation of structure and function of the parts of the carpel. We also aimed to find the potential pollinators and associate the morphology of the gynoecium with its role. We compare the characteristics of the gynoecium of T. usillo and discuss the carpel dimorphism with other genera within the Carolus clade in relation with their pollination syndromes. Carpels were processed according to classic techniques for scanning electron microscopy and bright field microscopy. We conducted field observation in different populations of T. usillo and captured the insects that were identified to specific level. The gynoecium of T. usillo shows inter-population and intra-individual variability. Some have three well-developed carpels, while most of them present two posterior carpels with differentiated styles and stigmas and the anterior one with a shorter style with or without stigma. The ovary has three locules with one ovule each. A compitum is formed and all ovules may be fecundated. However, fruits have generally one seed that develops in the anterior locule. Centris brethesi is the potential pollinator. The gynoecium of T. usillo reflects part of the variation in the carpel dimorphism that probably arose in the branch of the Carolus clade, and evolved in diverse ways in the lineages of this group. Tricomaria usillo seems to represent a recent transition towards reaching a stable form of carpel dimorphism and definitive division of labors of the carpels.






Similar content being viewed by others
References
Aliscioni SS, Torreta JP (2017) Malpighiaceae. Pages 163–205 in Flora Argentina, eds. Zuloaga FO, Belgrano MJ, Vol 17, Sigma, Buenos Aires
Aliscioni SS, Gotelli M, Torretta JP (2018) Structure of the stigma and style of Callaeum psilophyllum (Malpighiaceae) and its relation with potential pollinators. Protoplasma 255:1433–1442
Anderson WR (1979) Floral conservatism in neotropical Malpighiaceae. Biotropica 11:219–223
Anderson WR (1990) The origin of the Malpighiaceae. The evidence from morphology. Mem New York Bot Gard 64:210–224
Anderson WR (2006) Eight segregates from the neotropical genus Mascagnia (Malpighiaceae). Novon 16:168–204
Anderson WR, Anderson C, Davis CC (2006) Malpighiaceae. http://herbarium.lsa.umich.edu/malpigh/index.html [January 2019]
Armbruster WS, Debevec EM, Wilson MF (2002) Evolution of syncarpy in angiosperms: theoretical and phylogenetic analyses of the effects of carpel fusion on offspring quantity and quality. J Evol Biol 15:657–672
Arumuganathan K, Udaiyan K, Sugavanam V (1994) Structure and ontogeny of floral and extrafloral nectaries in Hiptage benghalensis (L.) Kurz (Malpighiaceae). Adv Plant Sci 7:105–111
Arumugasamy K, Inamdar JA, Subramanian RB (1989) Structure, ontogeny and secretion of oil secreting glands in Hiptage acuminate Wall. Curr Sci 58:260–261
Bailey IW, Swamy BGL (1951) The conduplicate carpel of dicotyledons and its initial trends of specialization. Am J Bot 38:373–379
Baum H (1948) Postgenitale Verwachsung in und zwischen Karpell- und Staubblattkreisen. Sitz ber Österr Akad Wiss Math-Natur-wiss Kl, Abt1 157:17–38
Cameron KM, Chase MW, Anderson WR, Hills HG (2001) Molecular systematics of Malpighiaceae: evidence from plastid rbcL and matK sequences. Amer J Bot 88:1847–1862
Chase MW (1981) A revision of Dicella (Malpighiaceae). Syst Bot 6:159–171
Davis CC, Anderson WR (2010) A complete generic phylogeny of Malpighiaceae inferred from nucleotide sequence data and morphology. Amer J Bot 97:2031–2048
Davis CC, Anderson WR, Donoghue MJ (2001) Phylogeny of Malpighiaceae: evidence from chloroplast ndhF and trnl-F nucleotide sequences. Amer J Bot 88:1830–1846
Davis CC, Fritsch PW, Bell CD, Mathews S (2004) High-latitude tertiary migrations of an exclusively tropical clade: evidence from Malpighiaceae. Int J Pl Sci 165:S107–S121
Davis CC, Schaefer H, Xi Z, Baum DA, Donoghue MJ, Harmon LJ (2014) Long-term morphological stasis maintained by a plant-pollinator mutualism. Proc National Acad Sci USA 111:5914–5919
Doyle JA, Endress PK (2000) Morphological phylogenetic analysis of basal angiosperms: comparison and combination with molecular data. Int J Plant Sci 161:121–153
Endress PK (1982) Syncarpy and alternative modes of escaping disadvantages of apocarpy in primitive angiosperms. Taxon 31:48–52
Endress PK (2001) The flowers in extant basal angiosperms and inferences on ancestral flowers. Int J Plant Sci 162:1111–1140
Endress PK (2011) Evolutionary diversification of the flowers in angiosperms. Am J Bot 98:370–396
Endress PK (2015) Patterns of Angiospermy development before carpel sealing across living angiosperms: diversity, and morphological and systematic aspects. Bot J Linn Soc 178:556–591
Endress PK, Doyle JA (2009) Reconstructing the ancestral flower and its initial specializations. Am J Bot 96:22–66
Endress PK, Igersheim A (2000) Gynoecium structure and evolution in basal angiosperms. Int J Plant Sci 161:211–223
Launert E (1968) Malpighiaceae. Flora of tropical East Africa. 24 pp.
Leme FM, Staedler YM, Schönenberger J, Teixeira SP (2018) Ontogeny and vascularization elucidate the atypical floral structure of Ampelocera glabra, a tropical species of Ulmaceae. Int J Plant Sci 179:461–476
Niedenzu FJ (1928) Malpighiaceae Pars I Pages 67–84 in Das Pflanzenreich, ed. A Engler. IV, 141
Qian ZN, Meng QW, Ren MX (2016) Pollination ecotypes and herkogamy variation of Hiptage benghalensis (Malpighiaceae) with mirror-image flowers. Biodivers Sci 24:1364–1372
Roig Alsina A (2000) Claves para las especies argentinas de Centris (Hymenoptera, Apidae), con descripción de nuevas especies y notas sobre distribución. Revista Mus Argent Ci Nat, N S 2:171–193
Siegel BA, Verbeke JA (1989) Diffusible factors essential for epidermal cell redifferentiaion in Catharanthus roseus. Science 244:580–582
Sigrist MR, Sazima M (2004) Pollination and reproductive biology of twelve species of Neotropical Malpighiaceae: stigma morphology and its implications for the breeding system. Ann Bot 94:33–41
Sokoloff DD, Nuraliev MS, Oskolski AA, Remizowa MV (2017) Gynoecium evolution in angiosperms: monomery, pseudomonomery, and mixomery. Mosc Univ Biol Sci Bull 72:97–108
Stebbins GL (1974) Flowering plants: evolution above the species level. Harvard University Press, Cambridge
Subramanian RB, Arumugasamy K, Inamdar JA (1990) Studies in the secretory glands of Hiptage sericea (Malpighiaceae). Nord J Bot 10:57–62
Thiers B (2014) Index Herbariorum: a global directory of public herbaria and associated staff. New York Botanical Garden’s Virtual Herbarium. Available from: http://sweetgum.nybg.org/ih (accessed December 2018)
Verbeke JA (1992) Fusion events during floral morphogenesis. Annual Rev Pl Biol 43:583–598
Vogel S (1990) History of the Malpighiaceae in the light of pollination ecology. Mem New York Bot Gard 55:130–142
Walker DB (1975) Postgenital carpel fusion in Catharanthus roseus (Apocynaceae). I. Light and scanning electron microscopic study of gynoecial ontogeny. Am J Bot 62:457–467
Wilczek R (1955) Novitates africanae I (Malpighiaceae et Linaceae). Bull Jard Bot État Bruxelles 25:303–313
Wilczek R (1959) Novitates africanae VI Flabellariopsis acuminata (Engl.) R. Wilczek descr. ampl. Bull Jard Bot État Bruxelles 29:193–194
Zarlavsky GE (2014) Histología Vegetal: técnicas simples y complejas. Sociedad Argentina de Botánica, Buenos Aires, Argentina
Zhang W, Kramer EM, Davis CC (2016) Differential expression of CYC2 genes and the elaboration of floral morphologies in Hiptage, an Old World genus of Malpighiaceae. Int J Plant Sci 177:551–558
Acknowledgments
We thank G. Zarvlasky for technical assistance; A. Calviño for providing material from La Cantina, Córdoba; R. Saurral for the revision of the English language; and two anonymous reviewers.
Funding
This work was funded by a research grant from Agencia Nacional de Promoción Científica y Tecnológica, grant number PICT 2013-1867 to S. Aliscioni, Consejo Nacional de Investigaciones Científicas y Técnicas, grant number PIP 11220110100312, and Universidad de Buenos Aires, grant number UBACyT 20020130200203BA to J. P. Torretta. Sandra Aliscioni, Marina Gotelli and Juan Pablo Torretta are affiliated with Consejo Nacional de Investigaciones Científicas y Técnicas, and Universidad de Buenos Aires, Argentina.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants performed by any of the authors.
Additional information
Handling Editor: Peter Nick
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Aliscioni, S.S., Gotelli, M. & Torretta, J.P. Gynoecium with carpel dimorphism in Tricomaria usillo, comparison with other genera of the Carolus clade (Malpighiaceae). Protoplasma 256, 1133–1144 (2019). https://doi.org/10.1007/s00709-019-01373-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-019-01373-3