Abstract
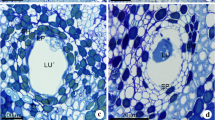
Protium heptaphyllum is a Burseraceae species known by the production of aromatic resin with medicinal, economic, and ecological values. Information on the development, architecture, and lifetime of the secretory system are crucial to understand the resin production and contribute to a more sustainable tapping regime. We investigated the histology and ultrastructure of the secretory canals under a developmental point of view. Stem samples were analyzed under light and transmission electron microscopy by conventional and cytochemical methods. Secretory canals, originated from procambium and cambium, occurred immersed in the primary and secondary phloem. Mature canals have a secretory epithelium and a wide lumen where the exudate is accumulated. A sheath of parenchyma cells with meristematic features surrounds the epithelium. The canals originate by schizogenesis and develop by schyzolysigenesis. Canals active in secretion occurred since the shoot apex and near the cambium. In the dilation zone of the secondary phloem, secretory canals exhibit sclerified epithelial and sheath cells and are inactive in secretion. Secreting epithelial cells have subcellular apparatus consistent with oleoresin, polysaccharides, and enzymes secretion. Pectinase and cellulase were cytochemically detected in developing canals and are involved in cell wall changes associated to canal growth and release of exudate. In P. heptaphyllum, the secretory system has a complex structure resultant from longitudinal growth, lateral ramification, and fusion of the adjacent canals, in addition to intrusive growth of both epithelial and sheath cells. Although some anatomical results are already known, ultrastructural data represent the novelty of this work. Our findings can contribute to the establishment of more efficient and sustainable techniques for resin extraction in this species.






Similar content being viewed by others
References
Allen RD, Nessler CL (1984) Cytochemical localization of pectinase activity in laticifers of Nerium oleander L. Protoplasma 119(1–2):74–78. https://doi.org/10.1007/BF01287819
Bal AK (1974) Cellulase. In: Hayat MA (ed) Electron microscopy of enzymes v3. Van Nostrand Reinhold, New York
Bosabalidis A, Tsekos I (1982a) Ultrastructural studies on the secretory cavities of Citrus deliciosa. Early stages of the gland cell differentiation. Protoplasma 112(1–2):55–62. https://doi.org/10.1007/BF01280215
Bosabalidis A, Tsekos I (1982b) Glandular scale development and essential oil secretion in Origanum dictamnus L. Planta 156(6):496–504. https://doi.org/10.1007/BF00392771
Bosshard HH, Hug UE (1980) The anastomoses of the resin canal system in Picea abies (L.) Karst., Larix decidua Mill. and Pinus sylvestris L. Holz Roh Werkst 38(9):325–328. https://doi.org/10.1007/BF02611082
Bowers WS, Evans PH, Venable DL, Becerra JX (2001) Interactions between chemical and mechanical defenses in the plant genus Bursera and their implications for herbivores. Am Zool 41:865–876
Brighigna L, Milocani E, Papini A, Vesprini JL (2006) Programmed cell death in the nucellus of Tillandsia (Bromeliaceae). Caryologia 59(4):334–339. https://doi.org/10.1080/00087114.2006.10797935
Bukatsch F (1972) Bemerkungen zur Doppelfárbung Astrablau-Safranin. Mikrokosmos 61:255
Canaveze Y, Machado SR (2015) Leaf colleters in Tabernaemontana catharinensis (Apocynaceae, Rauvolfioideae): structure, ontogenesis, and cellular secretion. Botany 93(5):287–296. https://doi.org/10.1139/cjb-2014-0229
Chamberlain CJ (1932) Methods in plant histology. The University of Chicago, Chicago
Cheniclet C, Carde JP (1985) Presence of leucoplasts in secretory cells and of monoterpenes in the essential oil: a correlative study. Isr J Bot 34:219–238
Citó AGL, Costa FB, Lopes JAD, Oliveira VMM, Chaves MH (2006) Identificação dos constituintes voláteis de frutos e folhas de Protium heptaphyllum Aubl (March). Revista Brasileira de Plantas Medicinais 8:4–7
Daly DC, Fine WA (2011) A new amazonian section of Protium (Burseraceae) including both edaphic specialist and generalista taxa. Studies in Neotropical Burseraceae XVI. Sistematic Bot 36(4):939–949. https://doi.org/10.1600/036364411X604958
Danon A, Delorme V, Mailhac N, Gallois P (2000) Plant programmed cell death: a common way to die. Plant Physiol Biochem 38(9):647–655
David R, Carde JP (1964) Coloration différentielle dês inclusions lipidique et ter-peniques des pseudophylles du pine maritime aumoyen du reactif Nadi Paris. CR Acad Sci Paris 257:1338–1340
Evert RF (2006) Esau’s plant anatomy. In: meristems, cells and tissues of the plant body their structure, function and development, 3rd edn. John Wiley and Sons, New Jersey. https://doi.org/10.1002/0470047380
Fahn A (1979) Secretory tissues in plants. Academic Press, London
Furr M, Mahlberg PG (1981) Histochemical analyses of lacticifers and glandular trichomes in Cannabis sativa. J Nat Prod 44(2):153–159. https://doi.org/10.1021/np50014a002
Gerrits PO (1991) The application of glycol methacrylate in histotechnology: some fundamental principles. Departament of Anatomy and Embryology State University of Gröningen, Gröningen
Johansen DA (1940) Plant microtechnique. McGraw-Hill, New York
Langenheim JH (2003) Plant resins: chemistry, evolution, ecology and ethnobotany. Timber Press, Portland
Lima TAAC, Ribeiro JELS, Marques MOM, Facanali R, Lima MP (2016) Estimulo para produção de resina em Protium hebetatum Daly e avaliação dos constituintes químicos voláteis. Scientia Amazonia 5:21–24
Liu P, Liang S, Yao N, Wu H (2012) Programmed cell death of secretory cavity cells in fruits of Citrus grandis cv. Tomentosa is associated with activation of caspase 3-like protease. Trees Struct Funct 26(6):1821–1835. https://doi.org/10.1007/s00468-012-0752-1
Lorenzi H (2002) Árvores brasileiras: manual de identificação e cultivo de plantas arbóreas do Brasil. Instituto Plantarum, Brasil
Machado SR, Carmello-Guerreiro SM (2001) Estrutura e desenvolvimento de canais secretores em frutos de Schinus terebinthifolius Raddi (Anacardiaceae). Acta Bot Bras 15(2):189–195. https://doi.org/10.1590/S0102-33062001000200005
Machado SR, Rodrigues TM (2004) Anatomia e ultra-estrutura do pulvino primário de Pterodon pubescens Benth. (Fabaceae-Faboideae). Rev Bras Bot 27(1):135–147. https://doi.org/10.1590/S0100-84042004000100015
Machado SR, Canaveze Y, Rodrigues TM (2017) Structure and functioning of oil cavities in the shoot apex of Metrodorea nigra A. St.-Hil. (Rutaceae). Protoplasma 254(4):1661–1674. https://doi.org/10.1007/s00709-016-1056-x
Marinho C, Teixeira S (2016) Cytochemical localization of pectinases and cellulases in developing laticifers of Maclura tinctoria and Ficus Montana (Moraceae). European Microscopy Congress: Proceedings
Matos FJA (1997) O Formulário Fitoterápico do professor Dias da Rocha, 2nd edn. UFC, Fortaleza
Mc Nair JB (1918) Secretory canals of Rhus diversiloba. Bot Gaz 65(3):268–273. https://doi.org/10.1086/332233
Metcalfe CR, Chalk L (1950) Anatomy of the dicotyledons leaves, stem and wood in relation to taxonomy with notes on economy uses. Clarendon press, Oxford
Nair GM, Patel KR, Subrahmanyam SV, Shah JJ (1981) Secretion of resin across the wall of the epithelial cell in the gum-resin canal of Comiphora mukul Engl. Ann Bot 47(3):419–421. https://doi.org/10.1093/oxfordjournals.aob.a086035
O’Brien TP, Feder N, McCully ME (1964) Polychromatic staining of plant cell walls by toluidine blue. Protoplasma 59(2):368–373. https://doi.org/10.1007/BF01248568
Papini A, Mosti S, van Doorn WG (2014) Classical macroautophagy in Lobivia rauschii (Cactaceae) and possible plastidial autophagy in Tillandsia albida (Bromeliaceae) tapetum cells. Protoplasma 251(3):719–725. https://doi.org/10.1007/s00709-013-0567-y
Paquet VE, Lessire R, Domergue F, Fouillen L, Filion G, Sedighi A, Charette SJ (2013) Lipid composition of multilamellar bodies secreted by dictyostelium discoideum reveals their amoebal origin. Eukaryot Cell 12 (10):1326–1334
Pearse AGE (1980) Histochemistry theoretical and applied, vol II, 4th edn. Longman Group Limited, London
Revilla J (2001) Plantas da Amazônia: oportunidades econômicas e sustentáveis. SEBRAE-AM/INSPA, Manaus
Reynolds ES (1963) The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol 17(1):208–212. https://doi.org/10.1083/jcb.17.1.208
Rodrigues TM, Machado SR (2012) Oil glands in Pterodon pubescens Benth. (Leguminosae-Papilionoideae): distribution, structure, and secretion mechanisms. Int J Plant Sci 173(9):984–992. https://doi.org/10.1086/667609
Rodrigues TM, Santos DC, Machado SR (2011a) The role of the parenchyma sheath and PCD during the development of oil cavities in Pterodon pubescens (Leguminosae-Papilionoideae). Comptes Rendus Biologies 334(7):535–543. https://doi.org/10.1016/j.crvi.2011.04.005
Rodrigues TM, Teixeira SP, Machado SR (2011b) The oleoresin secretory system in seedlings and adult plants of copaíba (Copaifera langsdorffii Desf., Leguminosae–Caesalpinioideae). Flora - Morphol Distribution Funct Ecol Plants 206(6):585–594. https://doi.org/10.1016/j.flora.2010.10.002
Siani AC, Ramos MFS, Menezes-de-Lima O, Soares ROA, Rosas EC, Susunaga GS, Guimarães AC, Zoghbi MGB, Henriques MGMO (1999) Evaluation of anti-inflamatory-related activity of essential oils from the leaves and resin of species of Protium. J Ethopharmacol 66(1):57–69. https://doi.org/10.1016/S0378-8741(98)00148-2
Siedlecka A, Wiklund S, Péronne MA, Micheli F, Lesniewska J, Sethson I, Edlund U, Richard L, Sundberg B, Mellerowicz EJ (2008) Pectin methyl esterase inhibits intrusive and symplastic cell growth in developing wood cells of Populus. Plant Physiol 146(2):554–565. https://doi.org/10.1104/pp.107.111963
Souza LR, Trindade FG, Oliveira RA, Costa LCB, Gomes VM, Cunha M (2016) Histochemical characterization of secretory ducts and essential oil analysis of Protium species (Burseraceae). J Essent Oil Res 28(2):166–171. https://doi.org/10.1080/10412905.2015.1092478
Sussunaga GS (1996) Estudo químico e biológico da resina produzida pela espécie Protium heptaphyllum March. (Burseraceae). Dissertation, University of Amazonas
Tolera M, Menger D, Sass-Klaassen U, Sterck FJ, Copini P, Bongers F (2013) Resin secretory structures of Boswellia papyrifera and applications for franckincense yield. Ann Bot 111(1):61–68. https://doi.org/10.1093/aob/mcs236
Turner GW, Gershenzon J, Nielson EE, Froehlich JE, Croteau RB (1999) Limonene synthase, the enzyme responsible for monoterpene biosynthesis in peppermint, is localized to leucoplasts of oil gland secretory cells. Plant Physiol 120(3):879–886. https://doi.org/10.1104/pp.120.3.879
van Doorn WG, Papini A (2016) Plastid degeneration in Tillandsia albida (Bromeliaceae) and Lobivia rauschii (Cactaceae) provides evidence about the origin and destiny of multilamellar bodies in plants. Phytomorphology 66:103–112
Acknowledgments
We thank Dr. Douglas C. B. Daly for the botanical identification, and the technical team of the Electron Microscopy Center, IBB UNESP, for assistance in processing the materials. FH Palermo (CNPq/Master) and J Nicolai (CNPq/PIBIC) received scholarship from Conselho Nacional de Desenvolvimento e Pesquisa, CNPq.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Handling Editor: Peter Nick
Electronic supplementary material
ESM 1
(GIF 801 kb)
Rights and permissions
About this article
Cite this article
Palermo, F.H., Rodrigues, M.I.d.A., de Nicolai, J. et al. Resin secretory canals in Protium heptaphyllum (Aubl.) Marchand. (Burseraceae): a tridimensional branched and anastomosed system. Protoplasma 255, 899–910 (2018). https://doi.org/10.1007/s00709-017-1197-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-017-1197-6