Abstract
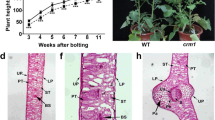
The chloroplast being an important organelle of plant cells could possibly be associated with plant cytoplasmic male sterility (CMS). To better understand the correlation between (CMS) and chloroplast, we presented a comprehensive analysis based on the changes of photosynthetic parameters, chloroplasts ultrastructure, soluble sugar and starch content, the relative expression of sugar and starch metabolism genes, and chloroplast genome in rice isonuclear alloplasmic CMS lines at the flowering stage. Leaf gas exchange parameters did not affect by CMS lines (M2BS and M2A), although intercellular CO2 concentration (C i) was influenced in both M2BS and M2A. Ultrastructural observation results indicated that many starch granules were observed in the chloroplast of CMS lines, especially bigger size in M2BS, while few ones in M2B. Only the chloroplasts of M2A contained some additional number of lipoids compared with those of the other two lines (M2B and M2BS). Soluble sugar and starch contents in CMS lines (M2BS and M2A) were significantly higher than those in maintainer line (M2B) (p < 0.01). The relative expression of sugar and starch metabolism genes indicated the imbalance of starch and sugar synthesis and decomposition may lead to accumulation of starch granules and demonstrated the presence of cytoplasmic effects. Moreover, chloroplast genome sequencing results showed similarity in both CMS lines, which revealed different single nucleotide polymorphisms (SNPs) and insertion/deletion (InDels) models compared with their maintainer line. Those models were located in psbD, rpoC2, rpl33, psbB, ndhA, ndhH, and intergenic regions. These findings, aligned with the possible association of CMS characteristics with cpDNA and genetically close relationship among both CMS lines, may contribute for future research.






Similar content being viewed by others
References
Bansal KC, Saha D (2012) Chloroplast genomics and genetic engineering for crop improvement. Agric Res 1(1):53–66. https://doi.org/10.1007/s40003-011-0010-6
Bernier G, Havelange A, Houssa C, Lejeunev P (1993) Physiological signals that induce flowering. Plant Cell 5(10):1147–1155. https://doi.org/10.1105/tpc.5.10.1147
Chen P, Mitsui T, Farmer D, Golovchenko J, Gordon R, Branton D (2004) Atomic layer deposition to fine-tune the surface properties and diameters of fabricated nanopores. Nano Lett 4(7):1333–1337. https://doi.org/10.1021/nl0494001
Doyle JJ, Doyle JL (1990) A rapid total DNA preparation procedure for fresh plant tissue. Focus 12:13–15
Eckardt NA (2006) Cytoplasmic male sterility and fertility restoration. Plant Cell 18(3):515–517. https://doi.org/10.1105/tpc.106.041830
Frankel R, Scoewcroft WR, Whitfeld PR (1979) Chloroplast DNA variation of isonuclear male sterile lines of Nicotiana. Mol Gen Genet 169:128–135
Fujii S, Toriyama K (2008) DCW11, down-regulated gene 11 in CW-type cytoplasmic male sterile rice, encoding mitochondrial protein phosphatase 2c is related to cytoplasmic male sterility. Plant cell physiol 49(4):633–640. https://doi.org/10.1093/pcp/pcn036
Galau GA, Wilkins TA (1989) Alloplasmic male sterility in AD allotetraploid Gossypium hissutum upon replacement of its resident A cytoplasm with that of D species G. harknessli. Thero Appl Genet 78(1):23–30. https://doi.org/10.1007/BF00299748
Gao J, Kong FR, Li JG (1987) Molecular cloning of specific fragments of cpDNA from male sterile line of rape. Acta Genet Sin 14:337–343
Guo JX, Liu YG (2009) The genetic and molecular basis of cytoplasmic male sterility and fertility restoration in rice. Chin Sci Bull 54(14):2404–2409. https://doi.org/10.1007/s11434-009-0322-0
Haveiange A, Bernier G (1983) Partia1 floral evocation by high irradiance in the long-day plant Sinapis alba. Physiol Plant 59(4):545–550. https://doi.org/10.1111/j.1399-3054.1983.tb06278.x
He GH, Hou L, Xiao YH, Luo XY, Niu GQ, Yang GW, Pei Y (2003) A common sequence difference between cytoplasmic male sterile lines and their maintainer lines existing in rice (Oryza sativa L.) chloroplast tRNA-Leu gene region. Euphytica 131(3):269–274. https://doi.org/10.1023/A:1024061919388
Hennen-Bierwagen TA, Liu F, Marsh R, Kim S, Gan Q, Tetlow IJ, Emes MJ, James MG, Myers AM (2008) Multiple starch biosynthetic enzymes from developing Zea mays endosperm associate in multisubunit complexes. Plant Physiology 146:1892–1908
Hennen-Bierwagen TA, Lin Q, Grimaud F, Planchot V, Keeling PL, James MG, Myers AM (2009) Proteins from multiple metabolic pathways associate with starch biosynthetic enzymes in high molecular weight complexes: a model for regulation of carbon allocation in maize amyloplasts. Plant Physiology 149:1541–1559
Hirose T, Zhang Z, Miyao A, Hirochika H, Ohsugi R, Terao T (2010) Disruption of a gene for rice sucrose transporter, OsSUT1, impairs pollen function but pollen maturation is unaffected. J Exp Bot 61(13):3639–3646. https://doi.org/10.1093/jxb/erq175
Hou L, Yang GW, He GH, Tang B, Xiao YH, Pei Y (2000) AFLP markers and sequence analysis in rice cytoplasmic male sterility line, Zhenshan 97A, and its maintainer line. Acta Bot Sin 42:591–594
Jiang PD, Zhu YG, Wang XL, Zhu W, Zhang XQ, Xie H, Wang XD (2007) Metabolism of reactive oxygen species in the cytoplasmic male-sterile cotton anther. Sci Agric Sin 40:244–249
Jiao J (2008) Comparative analysis of photosynthetic characteristics of different male sterile lines of wheat. Dissertation, Shandong Agricultural University
Jiao J, Gao QR, Zhang AM, Hao YY, Wang DW, Wang L, Qiu XM (2007) Diurnal changes of photosynthetic and physiological parameters in different male sterile lines of wheat. Acta Agron Sin 8:1267–1271
Lafitte HR, Ismail A, Bennett J (2009) Abiotic stress tolerance in rice for Asia: progress and the future. In: Fischer T, Turner N, Angus J, Mcintyre L, Robertson M, Borrell A et al (ed) New directions for a diverse planet: Proceedings for the 4th International Crop Science Congress. Brisbane
Li JG (1987) Chloroplast molecular genetics in higher plants. China agricultural university press, Beijing
Li H, Durbin R (2009) Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25(14):1754–1760. https://doi.org/10.1093/bioinformatics/btp324
Li JY, Li JG (1986) Chloroplast thylakoid member polypeptides and cytoplasmic male sterility. Acta Genet Sin 13:430–436
Li J, Yuan L (2010) Hybrid rice: genetics, breeding, and seed production. Plant breeding reviews. Wiley, New York, pp 15–158
Li B, Shen SX, Chen XP, Wang YH, Zhang CH (2006) Studies on the growth characters and photosynthesis in male sterile lines of eggplant during seedling stage. J Agric Univ HeBei 29:13–16
Li B, Shen SB, Chen XP, Luo SX, Wang YH, Guo LJ (2008) Comparative study on physiological and biochemical properties of male sterile lines and their maintainers in eggplant. J Plant Genet Resourc 1:46–50
Liu ZC (1986) Chloplast genome translated products and cytoplasmic male sterility. Acta Genet Sin 13:201–206
Liu YN, Li JG (1983) Chloroplast DNA and cytoplasmic male sterility. Acta Genet Sin 10:114–122
Liu YS, Wang XM, Wang WZ (1989) Structural variance analysis of mitochondrial CoI and CoII genes from normal and cytoplasmic male-sterile varieties of rice (Oryza sativa). Acta Genet Sin 15:348–354
Liu XL, Yu WW, Wang GB, Cao FL, Cai JF, Wang HL (2016) Comparative proteomic and physiological analysis reveals the variation mechanisms of leaf coloration and carbon fixation in a Xantha mutant of Ginkgo biloba L. Int J Mol Sci 17(12):1794. https://doi.org/10.3390/ijms17111794
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−△△Ct method. Methods 25:402–408
Lu Y (2010) Physiological characteristics and ultrastructure in male sterile lines of eggplant. Dissertation, Hebei Agriculture University
Luo JX, Ahmed R, Hashemi BK, Oscar Larroque O, Butardo JR, Tanner GJ, Colgrave ML, Upadhyaya NM, Tetlow IJ, Emes MJ, Millar A, Jobling SA, Morell MK, Li ZY (2015) The different effects of starch synthase IIa mutations or variation on endosperm amylose content of barley, wheat and rice are determined by the distribution of starch synthase I and starch branching enzyme IIb between the starch granule and amyloplast stroma. Theor Appl Genet 128(7):1407–1419. https://doi.org/10.1007/s00122-015-2515-z
McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, Garimella K, Altshuler D, Gabriel S, Daly M, DePristo MA (2010) The genome analysis toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res 20(9):1297–1303. https://doi.org/10.1101/gr.107524.110
Nakamura Y (2015) Biosynthesis of reserve starch. In: Nakamura Y (ed) Starch. Springer, Tokyo. https://doi.org/10.1007/978-4-431-55495-0_5
Neuhaus HE, Emes MJ (2000) Nonphotosynthetic metabolism in plastids. Annu Rev Plant Physiol Plant Mol Biol 51(1):111–140. https://doi.org/10.1146/annurev.arplant.51.1.111
Preiss J, Levi C (1979) Metabolism of starch in leaves. In: Gibbs M, Latzko E (eds) Photosynthesis II, Encyclopedia of plant physiology (new series), vol 6. Springer, Berlin. https://doi.org/10.1007/978-3-642-67242-2_24
Presis J (1991) Biology and molecular of starch synthesis and its regulation. Oxf Surv Plant Mol Cell Biol 7:59–114
Pryke JA, Bernler G (1987) Acid invertase activity in the apex of Sinapis alba during transition to flowering. Ann Bot 42:747–749
Qiao XL, Gao QR, Zhang AM, Zhao GQ, Liu ZB, Qiu XM (2006) Analysis of photosynthetic characteristics of K, V, T and CHA—cytoplasmic male sterile lines of wheat. Acta Agron Sin 9:1323–1328
Qiu ZM, Deng XH, Nie QJ, Zhu FJ (2008) SSR analysis of chloroplast DNA of pol cytoplasmic male sterile lines in Chinese cabbage. Hubei Agric Sci 47:134–137
Rehem BC, Almeida AAF, Santos IC, Gomes FP, Pirovani CP, Mangabeira PAO, Correa RX, Yamada RX, Valle RR (2011) Photosynthesis, chloroplast ultrastructure, chemical composition and oxidative stress in Theobroma cacao hybrids with the lethal gene Luteus-Pa mutant. Photosynthetica 49(1):127–139. https://doi.org/10.1007/s11099-011-0021-3
Scofield GN, Hirose T, Gaudron JA, Upadhyaya NM, Ohsugi R, Furbank RT (2002) Antisense suppression of the rice transporter gene, OsSUT1, leads to impaired grain filling and germination but does not affect photosynthesis. Funct Plant Biol 29:815–826
Shao RX, Xin LF, Zheng HF, Li LL, Ran WL, Mao J, Yang QH (2016) Changes in chloroplast ultrastructure in leaves of drought-stressed maize inbred lines. Photosynthetica 54(1):74–80. https://doi.org/10.1007/s11099-015-0158-6
Shen LY, Li RC, Wang RX (2012) Changes of protective enzyme activities in male sterile line 160S during fertility transformation. J Chongqing Normal Univ (Nat Sci) 29:82–86
Smidansky ED, Meyer FD, Blakeslee B, Weglarz TE, Greene TW, Giroux MJ (2007) Expression of a modified ADP-glucose pyrophosphorylase large subunit in wheat seeds stimulates photosynthesis and carbon metabolism. Planta 225(4):965–976. https://doi.org/10.1007/s00425-006-0400-3
Staehelin LA, Newcomb EH (2000) Membrane structure and membranous organelles. In: Buchanan RB, Gruissem W, Jones RL (eds) Biochemistry and molecular biology of plants. American Society of Plant Biology, Madison, pp 2–50
Sun XW, Tan YN, Sun ZZ, Yuan DY, Duan MJ (2014) Advances in sucrose transporters in rice. Life Sci Res 2:157–161
Tang DF, Chen P, Jin G, Li M, Wang CC, Bin ZL, Qian JH, Wang ZZ (2017) Identification of a novel cytoplasmic male sterile line M2BS induced by partial-length HcPDIL5-2atransformation in rice (Oryza sativa L.) J Plant Biol 60(2):146–153. https://doi.org/10.1007/s12374-016-0371-2
Tetlow IJ, Wait R, Lu Z, Akkasaeng R, Bowsher CG, Esposito S, Kosar-Hashemi B, Morell MK, Emes MJ (2004) Protein phosphorylation in amyloplasts regulates starch branching enzyme activity and protein-protein interactions. Plant Cell 16:694–708
Vedel F, Chetrit PC, Mathell (1985) Mitochondrial DNA polymorphism induced by protoplast fusion in Cruciferae. Theor Appl Genet 69:361–366
Wang L, Dong W, Zhou S (2012) Structural mutations and reorganizations in chloroplast genomes of flowering plants. Acta BotBoreal-Occident Sin 32:1282–1288
Wang AY, Zhang XH, Yang CH, Song ZJ, Du CQ, Chen DL, He YC, Cai DT (2013) Development and characterization of synthetic amphiploid (AABB) between Oryza sativa and Oryza punctata. Euphytica 189(1):1–8. https://doi.org/10.1007/s10681-012-0821-y
Wu X (2008) Prospects of developing hybrid rice with super high yield. Agron J 101:688–695
Wu LL, Wu J, Gong XD, Xu JL, Lin DZ, Dong YJ (2016) The rice pentatricopeptide repeat gene TCD10 is needed for chloroplast development under cold stress. Rice 9(1):67. https://doi.org/10.1186/s12284-016-0134-1
Xia H, Nelson MY, Donald B, Thompson DB, Guiltinan MJ (2011) Deficiency of maize starch-branching enzyme i results in altered starch fine structure, decreased digestibility and reduced coleoptile growth during germination. BMC Plant Biol 11(1):95–107. https://doi.org/10.1186/1471-2229-11-95
Xu ZM, Zhang EH, Chen YA, Ma QS (2006) Biochemical characteristics of the anthers and leaves of cytoplasmic male sterility line and its maintainer in cabbage. Acta Botan Boreal-Occident Sin 12:2592–2595
Xu M, Bai MY, Wei ST (2017) Changes in endogenous hormone between B. campestris ssp. Chinensis var. purpurea hort. CMS lines and their maintainer line at different development stages. Acta Agric Boreali-Occiden Sin 3:124–127
Yao YC, Wang SH, Kong Y (2007) Characteristics of photosynthesis machinism in different peach species under low light intensity. Sci Agric Sin 40:855–863
Zhang XZ (1992) Crop physiology research method. Agricultural Press, Beijing
Zhang B (2010) Studies on chloroplast genes genetic polymorphism of rice (Oryza sativa. L) and expression of chloroplast genes in isonuclear alloplasmic materials. Dissertation, Hunan Normal University
Zhang YM, Liang CY, Huang YW, Liu HX (1998) Comparison of respiratory pathways of CMS and its maintainer line rice (Oryza sativa L.) Acta Phytophysiol Sin 1:55–58
Zhang YJ, Zhao SY, Li YM (1999) Comparative study on translated products of chloroplast genome between the male sterility line in radish. Acta Hortic Sin 26:123–124
Zhang ZJ, Li HZ, Zhou WJ, Takeuchi Y, Yoneyama K (2006) Effect of 5-aminolevulinic acid on development and salt tolerance of potato (Solanum tuberosum L.) microtubers in vitro. Plant Growth Regul 49:27–34
Zhang YW, Wang ZY, Tian JH, Chen N, Dong YH, Chen WJ, Li DR, Zhao XG (2012) Photosynthesis of cytoplasmic male sterility lines with homocytonic and heteronuclear and their maintainers of rapeseed. Chin J oil Crop Sci 3:249–255
Zhao HY (2013) The mechanisms of male sterility on new CMS Yamian A in cotton. Dissertation, Shanxi Agriculture University
Zheng JG, Liu LH (1987) Studies on cytoplasmic effects in hybrid rice—investigation of ultra microscopic structure of chloroplast and mitochondrion. Sci Agric Sin 20:43–47
Zhou K, Si LT, Zhang Q (2007) The comparative research of the photosynthesis and the respiration of the CMS lines and the maintainer lines of radish. Northern. Horticulture 6:3–5
Funding
This study was supported by the National Natural Science Foundation of China (31171600, 31360348).
Author information
Authors and Affiliations
Contributions
TDF performed the experiments and wrote the manuscript. WF, MHK, and AK performed cytological observation and data analysis, and revised the manuscript. CP, LZQ, and SQQ compared the gas exchanges. JRX, ZL, LB, and XHY helped perform the determination of C/N ratio. ZRY designed and guided the experiments, and revised the manuscript. All the authors agreed on the contents of the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Jaideep Mathur
Electronic supplementary material
ESM 1
(DOCX 18 kb)
Rights and permissions
About this article
Cite this article
Tang, D., Wei, F., Kashif, M.H. et al. Analysis of chloroplast differences in leaves of rice isonuclear alloplasmic lines. Protoplasma 255, 863–871 (2018). https://doi.org/10.1007/s00709-017-1189-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-017-1189-6