Abstract
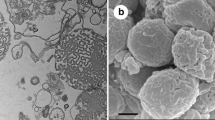
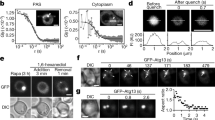
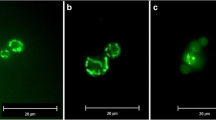
Cubic membranes (CM) are highly organized membrane structures found in biological systems. They are mathematically well defined and reveal a three-dimensional nano-periodic structure with cubic symmetry. These membrane arrangements are frequently induced in cells under stress, disease conditions, or upon viral infection. In this study, we investigated CM formation in the mitochondria of amoeba Chaos carolinense and observed a striking correlation between the organism’s ability to generate CM and the cell survival under starvation. Since starvation also induces autophagy, rapamycin was used to pharmacologically induce autophagy, and interestingly, CM formation was observed in parallel. Conversely, inhibition of autophagy reverted the cubic mitochondrial inner membrane morphology to tubular structure. In starved Chaos cells, mitochondria and autophagosomes did not co-localize and ATP production was sustained. CM transition in the mitochondria during starvation or upon induction of autophagy might prevent their sequestration by autophagosomes, thus slowing their rate of degradation. Such sustained mitochondrial activity may allow amoeba Chaos cells to survive for a longer period upon starvation.





Similar content being viewed by others
References
Almsherqi ZA, Kohlwein SD, Deng Y (2006) Cubic membranes: a legend beyond the Flatland* of cell membrane organization. J Cell Biol 173:839–844
Almsherqi ZA, Landh T, Kohlwein SD, Deng Y (2009) Chapter 6: cubic membranes the missing dimension of cell membrane organization. Int Rev Cell Mol Biol 274:275–342
Bruce DL, Marshall JM Jr (1965) Some ionic and bioelectric properties of the ameba Chaos chaos. J Gen Physiol 49:151–178
Chong K, Deng Y (2012) The three dimensionality of cell membranes: lamellar to cubic membrane transition as investigated by electron microscopy. Methods Cell Biol 108:319–343
Chong K, Tan OL, Almsherqi ZA, Lin Q, Kohlwein SD, Deng Y (2015) Isolation of mitochondria with cubic membrane morphology reveals specific ionic requirements for the preservation of membrane structure. Protoplasma 252:689–696
Deng Y, Mieczkowski M (1998) Three-dimensional periodic cubic membrane structure in the mitochondria of amoebae Chaos carolinensis. Protoplasma 203:16–25
Deng Y, Marko M, Buttle KF, Leith A, Mieczkowski M, Mannella CA (1999) Cubic membrane structure in amoeba (Chaos carolinensis) mitochondria determined by electron microscopic tomography. J Struct Biol 127:231–239
Deng Y, Almsherqi ZA, Ng MM, Kohlwein SD (2010) Do viruses subvert cholesterol homeostasis to induce host cubic membranes? Trends Cell Biol 20:371–379
Deter RL, De Duve C (1967) Influence of glucagon, an inducer of cellular autophagy, on some physical properties of rat liver lysosomes. J Cell Biol 33:437–449
Duszenko M, Ginger ML, Brennand A, Gualdron-Lopez M, Colombo MI, Coombs GH et al (2011) Autophagy in protists. Autophagy 7:127–158
Feng Y, Yao Z, Klionsky DJ (2015) How to control self-digestion: transcriptional, post-transcriptional, and post-translational regulation of autophagy. Trends Cell Biol 25:354–363
Fok AK, Allen RD (1979) Axenic Paramecium caudatum. I Mass culture and structure. J Protozool 26:463–470
Hughes T, Rusten TE (2007) Origin and evolution of self-consumption: autophagy. In: eukaryotic membranes and cytoskeleton. Adv Exp Med Biol 607:111–118
Klionsky DJ (2007) Autophagy: from phenomenology to molecular understanding in less than a decade. Nat Rev Mol Cell Biol 8:931–937
Klionsky DJ, Cregg JM, Dunn WA Jr, Emr SD, Sakai Y, Sandoval IV, Sibirny A, Subramani S, Thumm M, Veenhuis M, Ohsumi Y (2003) A unified nomenclature for yeast autophagy-related genes. Dev Cell 5:539–545
Klionsky DJ, Abdalla FC, Abeliovich H, Abraham RT, Acevedo-Arozena A, Adeli K et al (2012) Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy 8:445–544
Lamming DW, Ye L, Katajisto P, Goncalves MD, Saitoh M, Stevens DM, Davis JG, Salmon AB, Richardson A, Ahima RS, Guertin DA, Sabatini DM, Baur JA (2012) Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science 335:1638–1643
Lingwood D, Schuck S, Ferguson C, Gerl M, Simons K (2009a) Morphological homeostasis by autophagy. Autophagy 5:1039–1040
Lingwood D, Schuck S, Ferguson C, Gerl MJ, Simons K (2009b) Generation of cubic membranes by controlled homotypic interaction of membrane proteins in the endoplasmic reticulum. J Biol Chem 284:12041–12048
Marino G, Niso-Santano M, Baehrecke EH, Kroemer G (2014) Self-consumption: the interplay of autophagy and apoptosis. Nat Rev Mol Cell Biol 15:81–94
Mizushima N, Ohsumi Y, Yoshimori T (2002) Autophagosome formation in mammalian cells. Cell Struct Funct 27:421–429
Mizushima N, Kuma A, Kobayashi Y, Yamamoto A, Matsubae M, Takao T, Natsume T, Ohsumi Y, Yoshimori T (2003) Mouse Apg16L, a novel WD-repeat protein, targets to the autophagic isolation membrane with the Apg12-Apg5 conjugate. J Cell Sci 116:1679–1688
Morselli E, Maiuri MC, Markaki M, Megalou E, Pasparaki A, Palikaras K, Criollo A, Galluzzi L, Malik SA, Vitale I, Michaud M, Madeo F, Tavernarakis N, Kroemer G (2010) Caloric restriction and resveratrol promote longevity through the Sirtuin-1-dependent induction of autophagy. Cell Death Dis 1:e10
Otto GP, Wu MY, Kazgan N, Anderson OR, Kessin RH (2003) Macroautophagy is required for multicellular development of the social amoeba Dictyostelium discoideum. J Biol Chem 278:17636–17645
Papini A, van Doorn WG (2015) Crystalloids in apparent autophagic plastids: remnants of plastids or peroxisomes? J Plant Physiol 174:36–40
Powers RW 3rd, Kaeberlein M, Caldwell SD, Kennedy BK, Fields S (2006) Extension of chronological life span in yeast by decreased TOR pathway signaling. Genes Dev 20:174–184
Rigden DJ, Michels PA, Ginger ML (2009) Autophagy in protists: examples of secondary loss, lineage-specific innovations, and the conundrum of remodeling a single mitochondrion. Autophagy 5:784–794
Stromhaug PE, Klionsky DJ (2001) Approaching the molecular mechanism of autophagy. Traffic 2:524–531
Takeshige K, Baba M, Tsuboi S, Noda T, Ohsumi Y (1992) Autophagy in yeast demonstrated with proteinase-deficient mutants and conditions for its induction. J Cell Biol 119:301–311
Tan O-LL, Almsherqi ZA, Deng Y (2005) A simple mass culture of the amoeba Chaos carolinense: revisit. Protistology 4:185–190
Tatar M, Bartke A, Antebi A (2003) The endocrine regulation of aging by insulin-like signals. Science 299:1346–1351
Tekinay T, Wu MY, Otto GP, Anderson OR, Kessin RH (2006) Function of the Dictyostelium discoideum Atg1 kinase during autophagy and development. Eukaryot Cell 5:1797–1806
Terman A (1995) The effect of age on formation and elimination of autophagic vacuoles in mouse hepatocytes. Gerontology 41(Suppl 2):319–326
Vellai T, Takacs-Vellai K, Zhang Y, Kovacs AL, Orosz L, Muller F (2003) Genetics: influence of TOR kinase on lifespan in C. elegans. Nature 426:620
Yang Z, Klionsky DJ (2010) Mammalian autophagy: core molecular machinery and signaling regulation. Curr Opin Cell Biol 22:124–131
Yuan Y, Kadiyala CS, Ching TT, Hakimi P, Saha S, Xu H, Yuan C, Mullangi V, Wang L, Fivenson E, Hanson RW, Ewing R, Hsu A, Miyagi M, Feng Z (2012) Enhanced energy metabolism contributes to the extended life span of calorie-restricted Caenorhabditis elegans. J Biol Chem 287:31414–31426
Acknowledgements
We would like to thank Dr. Richard D. Allen and Dr. Masaki Ishida for providing Paramecium multimicronucleatum cell cultures, Professor Sepp D. Kohlwein, and Dr. Qingqiu Gong (College of Life Sciences, Nankai University, China) for critically reading the manuscript and providing helpful comments. We would also like to extend our gratitude to the Electron Microscopy Unit (Yong Loo Lin School of Medicine, National University of Singapore) for providing the facility for this work. This work was supported by grants from BMRC, Singapore (R-185-000-197-305), the National Natural Science Foundation of China (Grant No.: 31670841), and the Wenzhou Institute of Biomaterials and Engineering (Grant No.: WIBEZD2015010-02) to Yuru Deng.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Ralph Gräf
Electronic supplementary material
Figure S1.
Growth trend of amoeba Chaos with different feed (Para vs. Tetra). Growth rates were determined for amoeba cells cultivated on Paramecium (Para) or Tetrahymena (Tetra) by counting the number of amoeba in each culture dish at two-day interval (up to ten days). Data are presented as means ± S.D. of three independent experiments; the asterisk is considered significant with p<0.05. (GIF 281 kb).
Rights and permissions
About this article
Cite this article
Chong, K., Almsherqi, Z.A., Shen, HM. et al. Cubic membrane formation supports cell survival of amoeba Chaos under starvation-induced stress. Protoplasma 255, 517–525 (2018). https://doi.org/10.1007/s00709-017-1169-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-017-1169-x