Abstract
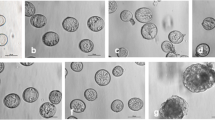
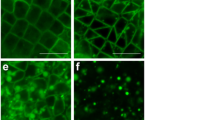
Most land plants have ill-defined microtubule-organizing centers (MTOCs), consisting of sites on the nuclear envelope or even along microtubules (MTs). In contrast, the spermatogenous cells of the pteridophyte Ceratopteris richardii have a well-defined MTOC, the blepharoplast, which organizes MTs through the last two division cycles. This allows a rare opportunity to study the organization and workings of a structurally well-defined plant MTOC. In this study, antheridial plants were treated with levels of oryzalin that cause complete MT loss from the cells containing blepharoplasts. The oryzalin was then washed out and plants were allowed to recover for varying amounts of time. If the spermatogenous cells were fixed prior to washing out, the blepharoplasts had an unusual appearance. In the matrix (pericentriolar) material where MT ends are normally found, clear areas of about the diameter of MTs were seen embedded in a much deeper matrix, made more obvious in stereo pairs. Occasionally, the matrix material was highly distended, although the basal body template cylinder morphology appeared to be unaltered. The blepharoplasts often occurred as clusters of 2 or 4, indicating that blepharoplast reproduction is not affected by the lack of MTs, but that their movement to the poles is. Gamma (γ) tubulin antibodies labeled the edge of the blepharoplast in areas where the pits are located, indicating that these might be sites for MT nucleation. After wash out, the new MTs always re-appeared on the blepharoplast and the recovery occurred within an hour of washout. MT lengths increased with increasing washout time and were indistinguishable from untreated blepharoplasts after 24 h of recovery. After washout, arrays formed in new sperm cells such as the spline and basal bodies were often malformed or present in multiple copies, as were the blepharoplasts in these cells prior to wash out. These data indicate that the blepharoplast serves as the site of MT nucleation and organization even after complete MT de-polymerization.






Similar content being viewed by others
References
Bornens M (1992) Structure and function of isolated centrosomes. In: Kalnins KI (ed) The centrosome. Academic, New York, pp 1–43
Brown RC, Lemmon BE (2007) The pleiomorphic plant MTOC: an evolutionary perspective. J Integr Plant Biol 49:1142–1153
Busby CH, Gunning BES (1989) Development of the quadripolar meiotic apparatus in Funaria spore mother cells: analysis by means of anti-microtubule drug treatments. J Cell Sci 93:267–277
Cleary A, Hardham AR (1988) Depolymerization of microtubule arrays in root tip cells by oryzalin, and their recovery with modified nucleation patterns. Can J Bot 66:2353–2366
Drykova D, Cenklova V, Sulimenko V, Volc J, Draber P, Binarova P (2003) Plant γ-tubulin interacts with αβ-tubulin dimmers and forms membrane associated complexes. Plant Cell 15:465–480
Erhardt M, Stoppin-Mellet V, Campagne S, Canaday J, Mutterer J, Fabian T, Sauter M, Muller T, Peter C, Lambert AM, Schmit AC (2002) The plant Spc98p homologue colocalizes with gamma-tubulin at microtubule nucleation sites and is required for microtubule nucleation. J Cell Sci 115:2423–2431
Hepler PK (1976) The blepharoplast of Marsilea: its de novo formation and spindle association. J Cell Sci 21:361–390
Hoffman JC, Vaughn KC (1995a) Post-translational tubulin modifications in spermatogenous cells of the pteridophyte Ceratopteris richardii. Protoplasma 186:169–182
Hoffman JC, Vaughn KC (1995b) Using developing spermatogenous cells of Ceratopteris to unlock the mysteries of the cytoskeleton. Int J Plant Sci 156:346–358
Hoffman JC, Vaughn KC (1996) Spline and flagellar microtubules are resistant to mitotic disrupter herbicides. Protoplasma 192:57–69
Hoffman JC, Vaughn KC, Joshi HC (1994) Structural and immunocytochemical characterization of microtubule organizing centers in pteridophyte spermatogenous cells. Protoplasma 179:46–60
Klink VP, Wolniak SM (2001) Centrin is necessary for the formation of the motile apparatus in spermatids of Marsilea. Mol Biol Cell 12:761–776
Klink VP, Wolniak SM (2003) Changes in the abundance and distribution of conserved centrosomal, cytoskeletal and ciliary proteins during spermiogenesis in Marsilea vestita. Cell Motil Cytoskel 56:57–73
Lambert AM (1993) Microtubule organizing centers in higher plants. Curr Opin Cell Biol 5:116–122
Liu B, Marc J, Joshi HC, Palevitz BA (1993) A gamma-tubulin-related protein associated with the microtubule arrays of higher plants in a cell cycle-dependent manner. J Cell Sci 104:1217–1228
Murata T, Sonobe S, Baskin TI, Hyodo S, Hasezawa S, Nagata T, Horio T, Hasebe M (2005) Microtubule-dependent microtubule nucleation based on recruitment of γ-tubulin in higher plants. Nature Cell Biol 7:961–968
Murata T, Tanahashi T, Nishiyama T, Yamaguchi K, Hasebe M (2007) How do plants organize microtubules without a centrosome. J Integr Plant Biol 49:1154–1163
Palevitz BA (1993) Morphological plasticity of the mitotic apparatus in plants and its developmental consequences. Plant Cell 5:1001–1009
Pennell RJ, Hyams JS, Bell PR (1986) The blepharoplast of Marsilea: a structure concerned with basal body assembly lacking tubulin. Eur J Cell Biol 40:238–241
Schmit AC (2002) Acentrosomal microtubule nucleation in higher plants. Int Rev Cytol 220:257–289
Shimamura M, Brown RC, Lemmon BE, Akashi T, Mizuno K, Nishihara N, Tomizawa KI, Yoshimoto K, Deguchi H, Hosoya H, Horio T, Mineyuki Y (2004) γ-Tubulin in basal land plants: characterization, localization, and implication in the evolution of acentriolar microtubule organizing centers. Plant Cell 16:45–59
Vaughn KC, Harper JD (1998) Microtubule-organizing-centers and nucleating sites in land plants. Int Rev Cytol 181:75–149
Vaughn KC, Sherman TD, Renzaglia KS (1993) A centrin homolog is a component of the multilayered structure in bryophytes and pteridophytes. Protoplasma 175:58–66
Acknowledgements
AJB was supported by a USDA-ARS Research Associate position to KCV. Initial studies of oryzalin treatment and recovery were performed by John Hoffman and Neil Durso. These were used to determine appropriate concentrations of oryzalin treatment and recovery time and are gratefully acknowledged here. Culturing of Ceratopteris was performed by Lynn Libous-Bailey and Brian Maxwell. H. Joshi generously provided the anti-γ tubulin antibody for use in these experiments. Mention of a trademark, proprietary product or vendor does not constitute an endorsement by the USDA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vaughn, K.C., Bowling, A.J. Recovery of microtubules on the blepharoplast of Ceratopteris spermatogenous cells after oryzalin treatment. Protoplasma 233, 231–240 (2008). https://doi.org/10.1007/s00709-008-0007-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-008-0007-6