Summary.
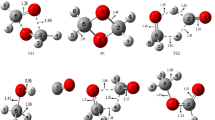
The complexes of formaldehyde and some of its derivatives with HF and HCl were investigated at HF/6-311 + +G** and MP2/6-311 + +G** levels of theory. Interaction energies were corrected for the basis set superposition error (BSSE). The full optimizations of dimers and monomers were performed during calculations. The Bader theory of atoms-in-molecules (AIM) was also applied for the localization of bond critical points (BCP) and for the calculation the electron densities and their Laplacians at these points. The relationships between H-bond energy and parameters obtained from calculations were also studied.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received June 29, 2001. Accepted (revised) October 29, 2001
Rights and permissions
About this article
Cite this article
Wiśniewska, M., Grabowski, S. Hydrogen Bonding Properties of the Complexes of Formaldehyde and its Derivatives with HF and HCl. Monatshefte fuer Chemie 133, 305–312 (2002). https://doi.org/10.1007/s007060200008
Issue Date:
DOI: https://doi.org/10.1007/s007060200008