Summary.
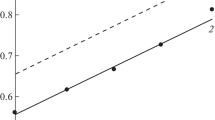
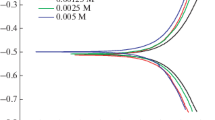
The inhibition of corrosion of iron in 2 M nitric acid and 2 M sulfuric acid solutions by substituted phenylhydantoin, thiohydantoin, and dithiohydantoin compounds was measured using thermometric, weight loss, and polarization methods. The three methods gave consistent results. The polarization curves indicated that the hydantoin compounds act as mixed-type inhibitors. The adsorption of the inhibitors were found to obey the Temkin adsorption isotherm. The higher inhibition efficiency of the additives in nitric with respect to sulfuric acid solution may be attributed to the reduced formation of soluble quaternary nitrogen salts in nitric acid medium, favouring adsorption of the parent additive on the metal surface. The obtained results indicate that the corrosion rate of iron in both acids increases with increasing temperature, both in absence and presence of the tested inhibitors. Kinetic-thermodynamic model functions and Temkin isotherm data are compared and discussed. The synergistic effect of halide anions on the inhibition efficiency of the hydantoin compounds was also investigated.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received June 5, 2000. Accepted (revised) September 13, 2000
Rights and permissions
About this article
Cite this article
Madkour, L., Hassanein, A., Ghoneim, M. et al. Inhibition Effect of Hydantoin Compounds on the Corrosion of Iron in Nitric and Sulfuric Acid Solutions. Monatshefte fuer Chemie 132, 245–258 (2001). https://doi.org/10.1007/s007060170134
Issue Date:
DOI: https://doi.org/10.1007/s007060170134