Summary.
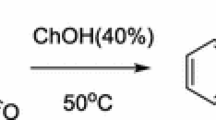
ω-Chloroalkyl biliverdins, prepared in three steps from 8-unsubstituted dipyrrinones in 70–80% overall yields, were reduced by sodium borohydride to provide the corresponding ω-chloroalkyl bilirubins in high yields. Upon treatment of the ω-chloroalkyl biliverdins with sodium hydroxide in ethanol, C12-N22 bridged biliverdins were obtained in high yields.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received December 13, 2000. Accepted January 15, 2001
Rights and permissions
About this article
Cite this article
Tu, B., Chen, Q., Yan, F. et al. Efficient Routes to ω-Chloroalkyl Bilirubins and C12-N22 Bridged Biliverdins. Monatshefte fuer Chemie 132, 693–705 (2001). https://doi.org/10.1007/s007060170084
Issue Date:
DOI: https://doi.org/10.1007/s007060170084