Summary.
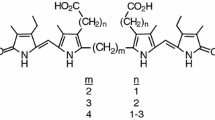
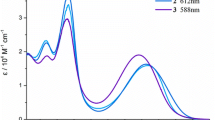
The first symmetrical bilirubin analog with CO2H groups replaced by SO3H, 8,12-bis-(2-sulfo-ethyl)-3,17-diethyl-2,7,13,18-tetramethyl-(10H,21H,23H,24H)-bilin-1,19-dione, was synthesized from methyl (2,4-dimethyl-5-ethoxycarbonyl-1H-pyrrol-3-yl) acetate in nine steps via the sulfonic acid analog of xanthobilirubic acid (XBR) and isolated as its disodium salt. The sulfonic acid group was introduced at an early stage of the synthesis by reaction of an intermediate, ethyl 4-(2-bromoethyl)-3,5-dimethyl-1H-pyrrole-2-carboxylate, with sodium sulfite. The disodium bilirubin disulfonate exhibits NMR spectroscopic properties rather similar to those of the parent carboxylic acid, mesobilirubin-XIIIα; however, its UV/Vis spectra are blue-shifted and broadened relative to those of the parent compound. Like mesobilirubin, the disulfonate displays a positive exciton chirality circular dichroism spectrum, albeit with weaker Cotton effects, in a buffered aqueous solution (pH = 7.4) containing a 2:1 molar ratio of human serum albumin.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received April 24, 2001. Accepted May 7, 2001
Rights and permissions
About this article
Cite this article
Boiadjiev, S., Lightner, D. A Water-Soluble Synthetic Bilirubin with Carboxyl Groups Replaced by Sulfonyl Moieties. Monatshefte fuer Chemie 132, 1201–1212 (2001). https://doi.org/10.1007/s007060170035
Issue Date:
DOI: https://doi.org/10.1007/s007060170035