Summary.
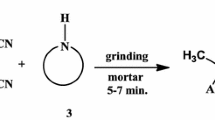
A simple, efficient, and eco-friendly procedure was developed for the condensation of N-methylhydroxylamine hydrochloride with benzaldehydes bearing electron-donating or electron-withdrawing substituents in the presence of powdered molecular sieves (3 Å) in a solventless system. α-Aryl-N-methylnitrones are obtained in excellent yields (80–100%); similar aromatic ketones do not react under these conditions, rendering the method chemoselective.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received March 26, 2001. Accepted (revised) May 7, 2001
Rights and permissions
About this article
Cite this article
Bigdeli, M., Nikje, M. An Efficient and Rapid Chemoselective Synthesis of α-Aryl-N-methylnitrones Dry Media. Monatshefte für Chemie 132, 1547–1549 (2001). https://doi.org/10.1007/s007060170011
Issue Date:
DOI: https://doi.org/10.1007/s007060170011