Summary.
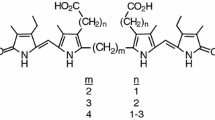
Analogs of bilirubin with vinyl groups replaced by symmetrically-disposed o-fluorophenyls (1, bis-exo, and 2, bis-endo) were synthesized and characterized spectroscopically. Their 1H NMR spectra and NOE data are consistent with an intramolecularly hydrogen-bonded ridge-tile conformation where each propionic acid group embraces an opposing dipyrrinone. Like bilirubin, 1 and 2 exhibit negative chirality induced circular dichroism (ICD) Cotton effects in chloroform containing quinine. Unlike bilirubin, however, in aqueous buffer containing human serum albumin, 2 exhibits a negative exciton chirality ICD, whereas that of 1 is positive.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received May 22, 2001. Accepted May 29, 2001
Rights and permissions
About this article
Cite this article
Brower, J., Lightner, D. Fluorophenyl Bilirubins: Synthesis and Stereochemistry. Monatshefte für Chemie 132, 1527–1546 (2001). https://doi.org/10.1007/s007060170010
Issue Date:
DOI: https://doi.org/10.1007/s007060170010