Summary.
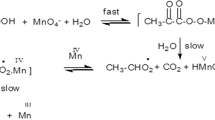
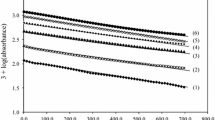
The kinetics of the oxidation of L-valine, (L-Val) by permanganate in aqueous alkaline medium at a constant ionic strength of 0.50 molċdm−3 was studied spectrophotometrically. The reaction is of first order in [permanganate ion] and of fractional order in both [L-Val] and [alkali]. Addition of products has no significant effect on the reaction rate. However, increasing ionic strength and decreasing dielectric constant of the medium increase the rate. The oxidation process in alkaline medium has been shown to proceed via two paths, one involving the interaction of L-valine with permanganate ion in a slow step to yield the products, and the other path the interaction of alkali with permanganate ion to give manganate. Some reaction constants involved in the mechanism were determined; calculated and observed rate constants agree excellently. The activation parameters were computed with respect to the slow step of the mechanism.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received December 30, 1999. Accepted (revised) March 6, 2000
Rights and permissions
About this article
Cite this article
Harihar, A., Kembhavi, M. & Nandibewoor, S. Kinetic and Mechanistic Study of the Oxidative Deamination and Decarboxylation of L-Valine by Alkaline Permanganate. Monatshefte fuer Chemie 131, 739–748 (2000). https://doi.org/10.1007/s007060070075
Issue Date:
DOI: https://doi.org/10.1007/s007060070075