Summary.
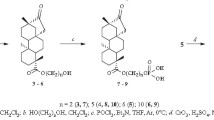
The bisquinolizidine carbinolamine 17-hydroxylupanine was synthesized de novo from lupanine using 2,3-dichloro-5,6-dicyano-1,4-benzoquinone; its structure was established by NMR techniques. The equatorial position of the hydroxy group as well as the prevailing boat form of ring C were determined. As expected, the carbinolamine converted into the C17=N16 anhydronium perchlorate upon treatment with HClO4. NMR analysis of the salt revealed a conformational equilibrium within rings A and D, whereas rings B and C remain rigid.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received March 13, 2000. Accepted March 21, 2000
Rights and permissions
About this article
Cite this article
Thiel, J., Boczoń, W. & Wysocka, W. A Stereostructural Study of 17-Hydroxylupanine and its Perchlorate. Monatshefte für Chemie 131, 1073–1081 (2000). https://doi.org/10.1007/s007060070040
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s007060070040