Abstract
A starch-supported cuprous iodide nanoparticles (CuI-NPs@starch) catalysed C–C bond cleaving reaction involving carbon-based leaving groups like malononitrile, ethyl cyanoacetate, acetylacetone, and Meldrum’s acid has been developed under moisture and air insensitive conditions. This competent C–C bond cleavage reaction is studied in detail as an attractive alternative method to synthesize biologically relevant bisindolylmethanes in moderate to good yields. The CuI-NPs@starch are synthesized in aqueous medium and characterized by X-ray powder diffraction, scanning electron microscopy, energy-dispersive X-ray spectroscopy studies.
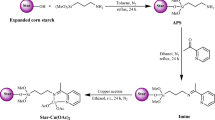
Graphical abstract



Similar content being viewed by others
Data availability
All relevant data are included in the manuscript and its supporting information.
References
Bhunia S, Das D (2022) Tetrahedron Lett 112:132738
Paul D, Chatterjee PN (2022) ChemistrySelect 7:e202200965
Chen F, Wang T, Jiao N (2014) Chem Rev 114:8613
Nakao Y (2021) Chem Rev 121:327
Mattalia JMR (2017) Beilstein J Org Chem 13:267
Dermenci A, Coe JW, Dong G (2014) Org Chem Front 1:567
Liu H, Feng M, Jiang X (2014) Chem Asian J 9:3360
Li H, Li W, Liu W, He Z, Li Z (2011) Angew Chem Int Ed 50:2975
Li W, Zheng X, Li Z (2013) Adv Synth Catal 355:181
Yang Y, Ni F, Shu WM, Wu AX (2014) Chem Eur J 20:11776
Wilsily A, Nguyen Y, Fillion E (2009) J Am Chem Soc 131:15606
Fillion E, Beaton E, Nguyen Y, Wilsily A, Bondarenko G, Jacq J (2016) Adv Synth Catal 358:3422
Mahoney SJ, Lou T, Bondarenko G, Fillion E (2012) Org Lett 14:3474
Vajargahy MP, Dabiri M, Bazgir A (2017) J Iran Chem Soc 14:1899
Paul D, Khatua S, Chatterjee PN (2019) New J Chem 43:10056
Paul D, Khatua D, Chatterjee PN (2018) ChemistrySelect 3:11649
Kalita G, Deka N, Paul D, Thapa L, Dutta GK, Chatterjee PN (2021) Synlett 32:304
Paul D, Chatterjee PN (2020) Eur J Org Chem 2020:4705
Lia M, Gu Y (2012) Adv Synth Catal 354:2484
Li M, Taheri A, Liu M, Sun S, Gu Y (2014) Adv Synth Catal 356:537
Li H, Yang J, Liu Y, Li Y (2009) J Org Chem 74:6797
Yang Q, Wang L, Guo T, Yu Z (2012) J Org Chem 77:8355
Chantana C, Jaratjaroonphong J (2021) J Org Chem 86:2312
Deb ML, Bhuyan PJ (2008) Synthesis 18:2891
Anilkumar G, Saranya S (2020) Copper catalysis in organic synthesis. Wiley-VCH, Weinheim
Prajapati JP, Das D, Katlakunta S, Maramu N, Ranjand V, Mallick S (2021) Inorg Chim Acta 515:120069
Prajapati JP, Toppo A, Majhi P, Pradhan U, Das A, Das D, Sriramulu G, Mallick S, Katlakunta S, Shukla AK (2023) ChemistrySelect 8:e202300531
Chakraborty N, Banerjee J, Chakraborty P, Banerjee A, Chanda S, Ray K, Acharya K, Sarkar J (2022) Green Chem Lett Rev 15:187
Kundu M, Mondal B, Das D, Roy UK (2022) ChemistrySelect 7:e202104543
Alonso F, Moglie Y, Radivoy G (2015) Acc Chem Res 48:2516
Gawande MB, Goswami A, Felpin F, Asefa T, Huang X, Silva R, Zou X, Zboril R, Varma RS (2016) Chem Rev 116:3722
Ojha NK, Zyryanov GV, Majee A, Charushin VN, Chupakhin ON, Santra S (2017) Coord Chem Rev 353:1
Das D (2016) ChemistrySelect 1:1959
Kute AD, Gaikwad RP, Warkad IR, Gawande MB (2022) Green Chem 24:3502
Wang S, Yuan M, Zhang Q, Huang S (2022) Curr Opin Green Sustainable Chem 38:100698
Pathak R, Punetha VD, Bhatt S, Punetha M (2024) J Mater Sci 59:6169
Shiri M, Zolfigol MA, Kruger HG, Tanbakouchian Z (2010) Chem Rev 110:2250
Mohapatra SS, Mukhi P, Mohanty A, Pal S, Sahoo AO, Das D, Roy S (2015) Tetrahedron Lett 56:5709
Mathavan S, Kannan K, Yamajala RBRD (2019) Org Biomol Chem 17:9620
Mallick S, Mukhi P, Kumari P, Mahato KR, Verma SK, Das D (2019) Catal Lett 149:3501
Ma Y, Gu M, Huang S, Liu X, Liu B, Ni C (2013) Mater Lett 100:166
Mousavi-Kamazani M, Zarghami Z, Salavati-Niasari M (2016) J Phys Chem C 120:2096
Xie Z-B, Sun D-Z, Jiang G-F, Le Z-G (2014) Molecules 19:19665
Yang J, Wang Z, Pan F, Lia Y, Bao W (2010) Org Biomol Chem 8:2975
Grigolo TA, Denofre S, Manarin F, Botteselle GV, Brandão P, Amaral AA, de Campos EA (2017) Dalton Trans 46:15698
Azizi N, Gholibeghlo E, Manocheri Z (2012) Sci Iran C 19:574
Hikawa H, Yokoyama Y (2013) RSC Adv 3:1061
Qu H-E, Xiao C, Wang N, Yu K-H, Hu Q-S, Liu L-X (2011) Molecules 16:3855
He Y-H, Cao J-F, Li R, Xiang Y, Yang D-C, Guan Z (2015) Tetrahedron 71:9299
Acknowledgements
The authors gratefully acknowledge the financial support from Science & Engineering Research Board (Grant CRG/2023/001214 & YSS/2015/001425).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Suresh, M., Singh, R.B., Katlakunta, S. et al. Starch-supported cuprous iodide nanoparticles catalysed C–C bond cleavage: use of carbon-based leaving groups for bisindolylmethane synthesis. Monatsh Chem (2024). https://doi.org/10.1007/s00706-024-03215-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00706-024-03215-2