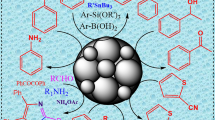
Abstract
In this study, we have focused on the preparation and application of a nano-ferric oxide catalyst as a heterogeneous catalyst in the one-pot, three-component synthesis of isoxazole derivatives. The ferric oxide catalyst was synthesized using a simple precipitation method and thoroughly characterized using techniques such as FT-IR, XRD, SEM, EDX, and BET. The effectiveness of the ferric oxide catalyst as a heterogeneous catalyst was investigated in the synthesis of 3,4,5-trisubstituted isoxazole. The results of the synthesis demonstrated that the catalyst exhibited high efficiency in facilitating the three-component, one-pot synthesis of 3,4,5-trisubstituted isoxazole derivatives. The synthesis route employed in this study offers several advantages, including its simplicity, ease of workup, and environmental friendliness. Furthermore, the catalyst was successfully recovered and reused for five cycles without experiencing a significant loss in catalytic activity. This finding highlights the excellent stability and promising potential of the catalyst for organic transformations. Overall, our study showcases the successful preparation and application of the nano-ferric oxide catalyst in the synthesis of isoxazole derivatives, providing valuable insights into its catalytic performance and potential for future applications in organic chemistry.
Graphical abstract









Similar content being viewed by others
Data availability
The spectroscopic data of the products are available in the supplementary information.
References
Kazemi M (2020) Synth Commun 50:1899
Kazemi M, Ghobadi M, Mirzaie A (2018) Nanotechnol Rev 7:43
Miceli M, Fontera P, Malara A (2021) Catalyst 11:591
Gawande MB, Branco PS, Varma RS (2013) Chem Soc Rev 42:3371
White RJ, Luque R, Budarine VL, Clark JH, MacQuarrie DJ (2009) Chem Soc Rev 38:481
Lim H, Lee J, Lee S, Kim J, Yoon J, Hyeon T (2006). Chem Commun. https://doi.org/10.1039/B513517F
Ma Y, Fu J, Gao Z, Zhang L, Li C, Wang T (2017) Catalysts 7:103
Tran PH-L, Tran TT-D, Vo TV, Lee BJ (2012) Arch Pharm Res 35:2045
Marnet S, Vasseur S, Grasset F, Veverka P, Goglio G, Demourgues A, Portier J, Pollert E, Duguet E (2006) Prog Solid State Chem 34:237
Schuth F, Lu A-H, Salabas EL (2007) Angew Chem Int Ed 46:1222
Lim CW, Lee IS (2010) Nano Today 5:412
Stevens PD, Li G, Fan J, Yen M, Gao Y (2005). Chem Commun. https://doi.org/10.1039/b505424a
Eskandari K, Khodabakhshi S (2018) Lett Org Chem 15:463
Wang Z, Xiao P, Shen B, He N (2006) Colloids Surf A Physicochem Eng Asp 276:116
Gnanaprakash G, Ayyappan S, Jayakumar T, Philip J, Raj B (2006) Nanotechnology 17:5851
Turtelli RS, Duong GV, Numes W, Grossinger R, Knobel M (2008) J Magn Magn Mater 320:e339
Cannas C, Ardu A, Musinu A, Peddis D, Piccaluga G (2008) Chem Mater 20:6364
Wu Q, Zhang H, Zhou L, Bao C, Zhu H, Zhang Y (2016) Chem Eng 67:484
Deng J, Chen YJ, Lu YA, Ma XY, Feng SF, Gao N, Li J (2017) Environ Sci Pollut Res 24:14396
Diao Z, Cheng L, Guo W, Hou X, Zheng P, Zhou Q (2021) Front Chem Sci Eng 15:643
Sharma R, Bansal S, Singhal S (2015) RSC Adv 5:6006
Abu-Diet AM, Abdel-Fatah SM (2018) Beni-Suef Univ J Basic Appl Sci 7:55
Sashkina K, Parkhomchuk E, Rudina N, Parmon V (2014) Microporus Mesoporous Mater 189:181
Barmade MA, Murumkar PR, Sharma MK, Yadav MR (2016) Curr Top Med Chem 16:2863
Ghasemi Z, Amale AH, Azizi S, Valizadeh S (2021) RSC Adv 11:36958
Galenko AV, Khebnikov AF, Novikov MS, Pakalnis VV, Rostovskii NV (2015) Russ Chem Rev 84:335
Walunj Y, Mhaske P, Kulkarni P (2021) Mini Rev Org Chem 18:55
Li J, Lin Z, Wu W, Jiang H (2020) Org Chem Front 7:2325
Serebryannikova AV, Galenko EE, Novikov MS, Khlebnikov AF (2019) J Org Chem 84:15567
Jensen MR, Schoepfer J, Radimerski T (2008) Breast Cancer Res 10:R33
Chen D, Shen A, Li J (2014) Eur J Med Chem 87:765
Sharp SY, Prodromou C, Boxall K (2007) Mol Cancer Ther 6:1198
Jin RY, Sun XH, Liu YF, Long W, Chen B, Shen SQ, Ma HX (2016) Spectrochim Acta Part A 152:226
Panda SS, Chowdary PVR, Jayashree BS (2009) Indian J Pharm Sci 71:684
Gutierrez M, Amiga J, Fuentes E, Palomo I, Astudillo L (2014) Platelets 25:234
Ali MA, Ismail R, Choon TS, Yoon YK, Pandian S, Ansari MZH (2011) J Enzyme Inhib Med Chem 68:343
Zhu J, Mo J, Lin H, Chen Y, Sun H (2018) Bioorg Med Chem 26:3065
Gaikwad NB, Bansod S, Mara A, Garise R, Srinivas N, Godugu C, Yaddanapudi VM (2021) Bioorg Med Chem 49:128294
Beyzaei H, Delijoo MK, Aryan R, Ghasemi B, Zahedi MM, Moghaddam M, Mahesh MM (2018) Chem Central J 12:114
Bormann AM, Morrison VA (2009) Drug Des Dev Ther 3:295
Waldo JP, Larock RC (2007) J Org Chem 72:9643
Oakdale JS, Sit RK, Fokin VV (2014) Chem Eur J 20:11101
Grecian S, Fokin VV (2008) Angew Chem Int Ed 47:8285
Hossain I, Khan HI, Kim SJ, Le HV (2022) Beilstein J Org Chem 18:446
Acknowledgements
The authors are thankful to the management and principal of PDEA's Ramkrishna More College Akurdi (Pune) for their encouragement, as well as the Central Instrumentation Facility (CIF) of Savitribai Phule Pune University and the SAIF CDRIL Lucknow for providing spectral data.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Totre, G., Shinde, D., Shirsath, S. et al. Ferric oxide nanocatalyst: synthesis, characterization, and application in the one-pot three-component synthesis of 3,4,5-trisubstituted isoxazole derivatives. Monatsh Chem (2024). https://doi.org/10.1007/s00706-024-03194-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00706-024-03194-4