Abstract
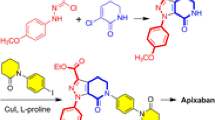
Apixaban is a highly potent, selective, and efficacious inhibitor of blood coagulation factor Xa. A practical and efficient process has been developed for the preparation of the key intermediate of apixaban. Starting from inexpensive 4-chloronitrobenzene and piperidine, an eight-step procedure for the intermediate has been developed. In this case, sodium chlorite is used twice to oxidize the piperidine cycle to the corresponding lactam under a CO2 atmosphere, resulting in the construction of two lactams. Furthermore, most of these reactions are highly efficient and practical as they occur under mild conditions. Most of the intermediates can be obtained through simple slurry or recrystallization, and column chromatography purification is not necessary.
Graphical abstract

Similar content being viewed by others
Data availability
All data and related metadata underlying the findings reported in a submitted manuscript should be deposited in an appropriate public repository, unless already provided as part of the submitted article.
References
Bauersachs R, Berkowitz SD, Brenner B, Buller HR, Decousus H, Gallus AS, Lensing AW, Misselwitz F, Prins MH, Raskob GE, Segers A, Verhamme P, Wells P, Agnelli G, Bounameaux H, Cohen A, Davidson BL, Piovella F, Schellong S (2010) N Engl J Med 363:2499
Roehrig S, Straub A, Pohlmann J, Lampe T, Pernerstorfer J, Schlemmer KH, Reinemer P, Perzborn E (2005) J Med Chem 48:5900
Nutescu E (2012) J Health-Syst Pharm 69:1113
Bauer KA (2011) J Thromb Haemost 9:12
Kvasnicka T, Malikova I, Zenahlikova Z, Kettnerova K, Brzezkova R, Zima T, Ulrych J, Briza J, Netuka I, Kvasnicka J (2017) Curr Drug Metab 18:636
Frost C, Nepal S, Wang J, Schuster A, Byon W, Boyd RA, Yu Z, Shenker A, Barrett YC, Mosqueda-Garcia R, LaCreta F (2013) Br J Clin Pharmacol 76:776
Byon W, Garonzik S, Boyd RA, Frost CE (2019) Clin Pharmacokinet 58:1265
Jiménez D, Yusen RD, Ramacciotti E (2012) Adv Ther 29:187
Buller H, Deitchman D, Prins M, Segers A (2008) J Thromb Haemost 6:1313
Top 200 Small Molecule Pharmaceuticals by Retail Sales, Compiled and Produced by the Njarðarson Group (The University of Arizona)
Brown DG, Wobst HJ (2021) J Med Chem 64:2312
Pinto DJP, Orwat MJ, Koch S, Rossi KA, Alexander RS, Smallwood A, Wong PC, Rendina AR, Luettgen JM, Knabb RM, He K, Xin B, Wexler RR, Lam PYS (2007) J Med Chem 50:5339
Mohan Rao D, Spandana D, Chandana Reddy J (2015) Novel process for the preparation of a lactam-containing compound. World patent WO2015177801A1, Nov 26, 2015; (2015) Chem Abstr 163:719211
Nevuluri NR, Rapolu RK, Iqbal J, Kandagatla B, Sen S, Dahanukar VH, Oruganti S (2017) Monatsh Chem 148:1477
Ji Y, Jiang J, Liu Q, Yu Y, Wang C, Liu A, Wang Y (2011) Process for preparing antithrombotic drug apixaban. Chinese patent CN101967145, Feb 9, 2011; (2011) Chem Abstr 154:284280
Jiang J, Ji Y (2013) Synth Commun 43:72
Maxwell BD, Tran SB, Chen SY, Zhang D, Chen B, Zhang H, Bonacorsi SJ Jr (2011) J Labelled Compd Radiopharm 54:418
Liu C, Yu T, Yang T, Sun H, Qin C, Jia Q, Chu C (2020) Org Process Res Dev 24:2633
Liu C, Sun H, Qin C, Yang T, Zhang W, Zhou Y, Li Y, Jia Z, Chu C (2022) Synlett 33:993
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21372081, 21172072).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dong, W., Gong, T., Liu, C. et al. A practical synthesis for the key intermediate of apixaban. Monatsh Chem 155, 99–104 (2024). https://doi.org/10.1007/s00706-023-03143-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-023-03143-7