Abstract
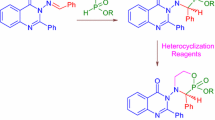
A preparatively convenient and efficient method is proposed for the synthesis of novel 1-oxo-1,2,3,4-tetrahydropyrrolo[1,2-a]pyrazine-8-carboxylic acids, based on the reaction of (3-oxopiperazine-2-ylidene)ethanoates with 2-bromo-1,1-diethoxyethane and accomplished through the stage of intermediate methyl 1-oxo-1,2,3,4-tetrahydropyrrolo[1,2-a]pyrazine-8-carboxylates, which were also isolated as individual compounds. A method of directly transforming 1-oxo-1,2,3,4-tetrahydropyrrolo[1,2-a]pyrazine-8-carboxylic acids into 1-oxo-N-(alkyl)aryl-1,2,3,4-tetrahydropyrrolo[1,2-a]pyrazine-8-carboxamides via the former’s interaction with aliphatic and aromatic amines in the presence of DIPEA and HATU was developed, with yields of 31–78%. A reliable structural determination of all the synthesized compounds has been performed by elemental analysis and a number of spectroscopic methods (1H and 13C NMR, HPLC/MS) as well as by X-ray diffraction analysis. Biological screening of all types of synthesized compounds revealed their moderate antibacterial and antifungal activity. The antioxidant effect level of the most active carboxamides was in the range of 59.3–74.5%, as compared to ascorbic acid (97.3%).
Graphical abstract






Similar content being viewed by others
Data availability
We declare that the data supporting the findings of this study are available within the paper and its Supplementary Information files. Should any raw data files be needed in another format, they are available from the corresponding author upon reasonable request.
References
Winant P, Horsten T, de Gil Melo SM, Emery F, Dehaen W (2021) Organics 2:118. https://doi.org/10.3390/org2020011
Uemoto H, Tsuda M, Kobayashi J (1999) J Nat Prod 62:1581. https://doi.org/10.1021/np9902542
Cafieri F, Fattorusso E, Mangoni A, Taglialatela-Scafati O (1995) Tetrahedron Lett 36:7893. https://doi.org/10.1016/0040-4039(95)01626-S
Li T, Wang N, Zhang T, Zhang B, Sajeevan TP, Joseph V, Armstrong L, He S, Yan X, Naman CB (2019) Mar Drugs 17:493. https://doi.org/10.3390/md17090493
Ebada SS, Linh MH, Longeon A, de Voogd NJ, Durieu E, Meijer L, Bourguet-Kondracki M-L, Singab ANB, Müller WEG, Proksch P (2015) Nat Prod Res 29:231. https://doi.org/10.1080/14786419.2014.947496
Cafieri F, Fattorusso E, Taglialatela-Scafati O (1998) J Nat Prod 61:122. https://doi.org/10.1021/np970323h
Scala F, Fattorusso E, Menna M, Taglialatela-Scafati O, Tierney M, Kaiser M, Tasdemir D (2010) Mar Drugs 8:2162. https://doi.org/10.3390/md8072162
Mancini I, Guella G, Amade P, Roussakis C, Pietra F (1997) Tetrahedron Lett 38:6271. https://doi.org/10.1016/S0040-4039(97)01405-6
Sun J, Wu J, An B, De Voogd NJ, Cheng W, Lin W (2018) Mar Drugs 16:9. https://doi.org/10.3390/md16010009
Mashiko T, Kumagai N, Shibasaki M (2008) Org Lett 10:2725. https://doi.org/10.1021/ol8008446
Meyers KM, Méndez-Andino J, Colson A-O, Hu XE, Wos JA, Mitchell MC, Hodge K, Howard J, Paris JL, Dowty ME, Obringer CM, Reizes O (2007) Bioorg Med Chem Lett 17:657. https://doi.org/10.1016/j.bmcl.2006.10.096
Ward RA, Bethel P, Cook C, Davies E, Debreczeni JE, Fairley G, Feron L, Flemington V, Graham MA, Greenwood R, Griffin N, Hanson L, Hopcroft P, Howard TD, Hudson J, James M, Jones CD, Jones CR, Lamont S, Lewis R, Lindsay N, Roberts K, Simpson I, St-Gallay S, Swallow S, Tang J, Tonge M, Wang Z, Zhai B (2017) J Med Chem 60:3438. https://doi.org/10.1021/acs.jmedchem.7b00267
Casuscelli F, Ardini E, Avanzi N, Casale E, Cervi G, D’Anello M, Donati D, Faiardi D, Ferguson RD, Fogliatto G, Galvani A, Marsiglio A, Mirizzi DG, Montemartini M, Orrenius C, Papeo G, Piutti C, Salom B, Felder ER (2013) Bioorg Med Chem 21:7364. https://doi.org/10.1016/j.bmc.2013.09.054
Micheli F, Cavanni P, Di Fabio R, Marchioro C, Donati D, Faedo S, Maffeis M, Sabbatini FM, Tranquillini ME (2006) Bioorg Med Chem Lett 16:1342. https://doi.org/10.1016/j.bmcl.2005.11.049
Fisher TE, Kim B, Staas DD, Lyle TA, Young SD, Vacca JP, Zrada MM, Hazuda DJ, Felock PJ, Schleif WA, Gabryelski LJ, Anari MR, Kochanskyd CJ, Wai JS (2007) Bioorg Med Chem Lett 17:6511. https://doi.org/10.1016/j.bmcl.2007.09.086
Piltan M, Moradi L, Abasi G, Zarei SA, Wolfe JP (2013) Beilstein J Org Chem 9:510. https://doi.org/10.3762/bjoc.9.55
Piltan M (2016) J Chem Res 40:410. https://doi.org/10.3184/174751916X14652279155994
Moradi L, Piltan M, Rostami H, Abasi G (2013) Chin Chem Lett 24:740. https://doi.org/10.1016/j.cclet.2013.04.038
Alizadeh A, Abadi MH, Ghanbaripour R (2014) Synlett 25:1705. https://doi.org/10.1055/s-0034-1378275
Weiss MM, Zheng X (2021) IRAK degraders and uses thereof. World Patent WO2021158634 A1 (2021) Chem Abstr 176: 10869-10870
Le Diguarher T, Casara P, Starck J-B, Henlin J-M, Davidson JEP, Murray JB, Graham CJ, Chen I-J, Geneste O, Hickman J, Depil S, Le Tiran A, Nyerges M, De Nanteuil G (2015) Indolizine compounds, a process for their preparation and pharmaceutical compositions containing them. United States Patent US20150051189A1 2015; (2013) Chem Abstr 159:290274
Gupta AK, Chakrasali RT, Ila H, Junjappa H (1989) Synthesis 1989:141. https://doi.org/10.1055/s-1989-27179
Barun O, Chakrabarti S, Ila H, Junjappa H (2001) J Org Chem 66:4457. https://doi.org/10.1021/jo010273s
Fan M-J, Li G-Q, Liang Y-M (2006) Tetrahedron 62:6782. https://doi.org/10.1016/j.tet.2006.04.100
Nami N, Neumuller B, Heravi MM, Haghdadi M (2008) Mendeleev Commun 18:153. https://doi.org/10.1016/j.mencom.2008.05.014
Choudhary G, Peddinti RK (2011) Green Chem 13:3290. https://doi.org/10.1039/C1GC15701A
Kawahara N, Shimamori T, Itoh T, Takayanagi H, Ogura H (1987) Chem Pharm Bull 35:457. https://doi.org/10.1248/cpb.35.457
Kawahara N, Nakajima T, Itoh T, Ogura H (1983) Heterocycles 20:121. https://doi.org/10.3987/r-1983-01-0121
Horsten T, Alegbejo Price TO, Van Meervelt L, da Silva EF, Dehaen W (2022) New J Chem 46:2028. https://doi.org/10.1039/D1NJ04965H
de Figueiredo RM, Suppo J-S, Campagne J-M (2016) Chem Rev 116:12029. https://doi.org/10.1021/acs.chemrev.6b00237
Massolo E, Pirola M, Benaglia M (2020) Eur J Org Chem 2020:4641. https://doi.org/10.1002/ejoc.202000080
Santos AS, Silva AMS, Marques MMB (2020) Eur J Org Chem 2020:2501. https://doi.org/10.1002/ejoc.202000106
Lundberg H, Tinnis F, Selander N, Adolfsson H (2014) Chem Soc Rev 43:2714. https://doi.org/10.1039/C3CS60345H
Carey JS, Laffan D, Thomson C, Williams MT (2006) Org Biomol Chem 4:2337. https://doi.org/10.1039/B602413K
Ghose AK, Viswanadhan VN, Wendoloski JJ (1999) J Comb Chem 1:55. https://doi.org/10.1021/cc9800071
Carpino LA (1993) J Am Chem Soc 115:4397. https://doi.org/10.1021/ja00063a082
Bhatt V, Samant SD, Pednekar S (2017) Lett Org Chem 14:764. https://doi.org/10.2174/1570178614666170710095437
Zefirov NS, Palyulin VA, Dashevskaya EE (1990) J Phys Org Chem 3:147. https://doi.org/10.1002/poc.610030304
Burgi H-B, Dunitz JD (1994) Structure correlation, vol 2. VCH, Weinheim, p 741
El-Hameed RHA, Sayed AI, Ali SM, Mosa MA, Khoder ZM, Fatahala SS (2021) J Enzyme Inhib Med Chem 36:2183. https://doi.org/10.1080/14756366.2021.1984904
Mohamed MS, Fathallah SS (2014) Mini-Rev Org Chem 11:477. https://doi.org/10.2174/1570193x113106660018
Choudhary D, Garg S, Kaur M, Sohal HS, Malhi DS, Kaur L, Verma M, Sharma A, Mutreja V (2023) Polycycl Aromat Compd 43:4512. https://doi.org/10.1080/10406638.2022.2092873
Miyazawa T, Takabatake T, Hasegawa M (1997) J Pharm Soc Japan 117:126. https://doi.org/10.1248/yakushi1947.117.2_126
El-Bayouki KAM, Basyouni WM, Mostafa EA (2010) Collect Czech Chem Commun 75:813. https://doi.org/10.1135/cccc2009566
Andreou D, Essien NB, Pubill-Ulldemolins C, Terzidis MA, Papadopoulos AN, Kostakis GE, Lykakis IN (2021) Org Lett 23:6685. https://doi.org/10.1021/acs.orglett.1c02251
Pontiki E, Hadjipavlou-Litina D, Patsilinakos A, Tran TM, Marson CM (2015) Future Med Chem 7:1937. https://doi.org/10.4155/fmc.15.104
Lima RN, Gonçalves JR, Silva VR, de Santos L, Bezerra DP, Soares MBP, Leitão A, Porto ALM (2020) Curr Bioact Compd 16:900. https://doi.org/10.2174/1573407215666190318144105
Nazarchuk OA (2016) Klin Khir 9:59. PMID: 30265488
Crowley PD, Gallagher HC (2014) J Appl Microbiol 117:611. https://doi.org/10.1111/jam.12554
Brand-Williams W, Cuvelier ME, Berset C (1995) LWT Food Sci Technol 28:25. https://doi.org/10.1016/S0023-6438(95)80008-5
Sheldrick GM (2008) Acta Crystallogr Sect A 64:112. https://doi.org/10.1107/S0108767307043930
Kowalska-Krochmal B, Dudek-Wicher R (2021) Pathogens 10:165. https://doi.org/10.3390/pathogens10020165
Acknowledgements
We are grateful to Enamine Ltd (Kyiv, Ukraine) for support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Litvinchuk, M.B., Bentya, A.V., Grozav, A.M. et al. Synthesis, antimicrobial and antioxidant activity of novel 1-oxo-1,2,3,4-tetrahydropyrrolo[1,2-a]pyrazine-8-carboxylic acids, esters, and amides thereof. Monatsh Chem 154, 1145–1159 (2023). https://doi.org/10.1007/s00706-023-03118-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-023-03118-8