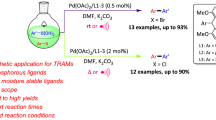
Abstract
A method for the selective formation of C2 coupling products via palladium-catalyzed cross-coupling of aryl titanium reagents and dihalogenated benzophenone or dihalogenated acetophenone scaffolds is described. The method uses Pd(dba)2 as catalyst and 2-diisopropylphosphino-2′-dimethylaminobiphenyl as ligand in THF/NMP (3:1) (4.0 cm3) for 10 h at 0 °C. The system can tolerate various functional groups such as aldehydes, esters, etc. In the process of optimizing the coupling method, we also synthesized two new ligands and produced five new monosubstituted aromatic ketones, which appear for the first time in this paper.
Graphical abstract

Similar content being viewed by others
Data availability
All relevant data are within the manuscript and its additional files.
References
Purser S, Moore PR, Swallow S, Gouverneur V (2008) Chem Soc Rev 37:320
Wang J, Sánchez-Roselló M, Acena JL, del Pozo C, Sorochinsky AE, Fustero S, Soloshonok VA, Liu H (2014) Chem Rev 114:2432
Magano J, Dunetz JR (2013) Transition metal-catalyzed couplings in process chemistry: case studies from the pharmaceutical industry. Wiley, Hoboken
Colacot T (2015) New trends in cross-coupling: theory and applications. RSC catalysis series, Cambridge
Wang JR, Manabe K (2009) Synthesis 1405
Hassan Z, Patonay T, Langer P (2013) Synlett 24:412
Heinrich ACJ, Thiedemann B, Gates PJ, Staubitz A (2015) Org Lett 15:4666
Montoir D, Tonnerre A, Duflos M, Bazin MA (2014) Eur J Org Chem 2014:14879
Singh R, Just G (1989) J Org Chem 54:4453
Newman SG, Lautens M (2010) J Am Chem Soc 132:11416
Manabe K, Yamaguchi M (2014) Catalysts 4:307
Yamaguchi M, Manabe K (2016) Topics Ccurr Chem 372:1
Dobrounig P, Trobe M, Breinbauer R (2017) Monatsh Chem 148:3
Yang W, Wang Y, Corte JR (2003) Org Lett 5:3131
Li GY (2002) Catalysis using phosphine oxide and phosphine sulfide complexes with Pd and Ni for the synthesis of biaryls and arylamines. Patent WO2002000574A2, Jan 03, 2002. Chem Abstr 136:85654
Houpis IN, Huang C, Nettekoven U, Chen JG, Liu R, Canters M (2008) Org Lett 10:5601
Ishikawa S, Manabe K (2010) Angew Chem Int Ed 49:772
Jin L, Xin J, Huang Z, He J, Lei A (2010) J Am Chem Soc 132:9607
Arakawa Y, Wada O, Yu TH (1981) Toxicol Appl Pharmacol 60:1
Shen HC, Crawley MJ, Trost BM (2012) In applications of transition metal catalysis in drug discovery and development: an industrial perspective, chapter 2, p 25. Wiley, Hoboken
Han JW, Tokunaga N, Hayashi T (2002) Synlett 871
Yang HT, Zhou S, Chang FS, Chen CR, Gau HM (2009) Organometallics 28:5715
Weber B, Seebach D (1994) Tetrahedron 50:7473
Lee HW, Lam FL, So CM, Lau CP, Chan ASC, Kwong FY (2009) Angew Chem Int Ed 48:7436
Andrii V, Mark G (2019) J Am Chem Soc 141:10994
Lee HW, So CM, Yuen OY, Wong WT, Kwong FY (2020) Org Chem Front 7:926
He XY (2021) Monatsh Chem 152:823
Korn TJ, Schade MA, Cheemala MN, Wirth S, Guevara SA, Cahiez G, Knochel P (2006) Synthesis 21:3547
He B, Nie H, Chen L, Lou XD, Hu RR, Qin AJ, Zhao ZJ, Tang BZ (2015) Org Lett 17:6174
Zeng XH, Xu DQ, Miao CX, Xia CG, Sun W (2014) RSC Adv 4:46494
Gallistl C, Proctor K, Bader K, Vetter W (2017) Environ Sci Pollut R 24:16815
Kim IK, Li X, Ullah MJ, Shaw PE, Wawrzinek R, Namdas EB, Lo SC (2015) Adv Mater 27:6390
Thom I, Besson C, Kleine T, Bolm C (2013) Angew Chem Int Ed 52:7509
Fernandes RA, Ramakrishna GV, Bethi V (2020) Org Biomol Chem 18:6115
Li J, Ye DJ, Liu H, Luo XM, Jiang HL (2008) Synth Commun 38:567
Bedford RB, Brenner PB, Carter E, Clifton J, Cogswell PM, Gower NJ, Haddow MF, Harvey JN, Kehl JA, Murphy DM, Neeve EC, Neidig ML, Nunn J, Snyder BER, Taylor J (2014) Organometallics 33:5767
Aranyos A, Old DW, Kiyomori A, Wolfe JP, Sadighi JP, Buchwald SL (1999) J Am Chem Soc 121:4369
Han C, Buchwald SL (2009) J Am Chem Soc 131:7532
Tomori H, Fox JM, Buchwald SL (2000) J Org Chem 65:5334
Zhou ZH, Zhang YQ, Xia W, Chen HX, Liang H, He XF, Yu SF, Cao RH, Qiu LQ (2016) Asian J Org Chem 5:1260
Rasovskiy A, Knochel P (2004) Angew Chem Int Ed 43:3333
Krasovskiy A, Knochel P (2006) Synthesis 5:890
Acknowledgements
The author gratefully acknowledge the financial (YX202215) support from Hebei Chemical & Pharmaceutical College.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, H. Site-selective cross-coupling of dihalogenated acetophenones and dihalogenated benzophenones with aryl titanium reagents under catalyst control. Monatsh Chem 154, 873–885 (2023). https://doi.org/10.1007/s00706-023-03101-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-023-03101-3