Abstract
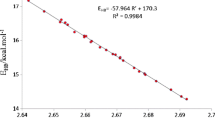
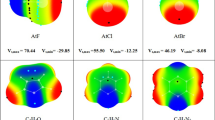
The present article demonstrates a quantum chemical approach based on the Quantum Theory of Atoms-In-Molecules (QTAIM) to assess the O−H⋅⋅⋅O intramolecular hydrogen-bonding (IMHB) interactions in a series of phenalene derivatives, namely, 9-hydroxy-2,4-dihydro-1H-phenalen-1-one (9HP1O), 2-hydroxy-9,9a-dihydro-1H-phenalen-1-one (2HP1O), and 3-hydroxy-1H-phenalen-2(4H)-one (3HP2O). The topological parameters and IMHB energies have been calculated based on density functional theory (using B3LYP and CAM-B3LYP hybrid functionals, and M06-2X and LC-ωPBE functionals) and Møller-Plesset perturbation theory (MP2) approaches. The calculated geometrical and topological parameters along with the IMHB energies show the different degrees of covalence in the IMHB interactions in the studied molecular structures, and thus reveal the inequivalence of substitution pair positions in the studied phenalene derivatives. The results derived from QTAIM analyses of the studied molecules are further corroborated from noncovalent interaction analysis including a visual portrayal of the noncovalent interactions.
Graphical abstract






Similar content being viewed by others
Data availability
None.
References
Eskandari K, Alsenoy CV (2014) J Comput Chem 35:1883
Bader RFW (2009) J Phys Chem A 113:10391
Bader RFW (1990) Atoms in molecules: a quantum theory. Oxford University Press, Oxford
Bader RFW (1991) Chem Rev 91:893
Matta CF, Boyd RJ (2007) The quantum theory of atoms in molecules. Wiley-VCH, Weinheim
Matta CF, Hernández-Trujillo J, Tang TH, Bader RFW (2003) Chem Eur J 9:1940
Hernández-Trujillo J, Matta CF (2007) Struct Chem 18:849
Eskandari K (2012) J Mol Model 18:3481
Pakiari A, Eskandari K (2007) J Mol Struct (Theochem) 806:1
Ganguly A, Paul BK, Guchhait N (2017) Comput Theor Chem 1117:108
Paul BK (2019) J Phys Org Chem 32:1
Paul BK, Guchhait N (2013) Chem Phys 412:58
Ganguly A (2021) Struct Chem 32:431
Bofill JM, Olivella S, Solé A, Anglada JM (1999) J Am Chem Soc 121:1337
Bach A, Lentz D, Luger P (2001) J Phys Chem A 105:7405
Matta CF, Castillo N, Boyd RJ (2005) J Phys Chem A 109:3669
Yanai T, Tew DP, Handy NC (2004) Chem Phys Lett 393:51
Chai JD, Head-Gordon M (2008) Phys Chem Chem Phys 10:6615
Biegler-König F, Schönbohm J, Bayles D (2001) J Comput Chem 22:524
Cioslowski J, Mixon ST, Edwards WD (1991) J Am Chem Soc 113:1083
Cioslowski J, Mixon ST (1992) J Am Chem Soc 114:4382
Jeffrey GA (1997) An introduction to hydrogen bonding. Oxford University Press, New York
Desiraju GR, Steiner T (1999) The weak hydrogen bond in structural chemistry and biology. Oxford University Press, New York
Grabowski SJ (2006) Hydrogen bonding - new insights. Series: Leszczynski J (ed), Challenges and advances in computational chemistry and physics. Springer, New York
Perrin CL, Nielson JB (1997) Annu Rev Phys Chem 48:511
Gerlt JA, Kreevoy MM, Cleland WW, Frey PA (1997) Chem Biol 4:259
Głowacki ED, Irimia-Vladu M, Bauer S, Sariciftci NS (2013) J Mater Chem B 1:3742
Paul BK, Mahanta S, Singh RB, Guchhait N (2010) J Phys Chem A 114:2618
Bader RFW (1985) Acc Chem Res 18:9
Bader RFW (2010) J Phys Chem A 114:7431
Popelier PLA (1998) J Phys Chem A 102:1873
Lazzeretti P (2004) Phys Chem Chem Phys 6:217
Grabowski SJ (2001) J Phys Chem A 105:10739
Grabowski SJ (2011) Chem Rev 111:2597
Grosch AA, van der Lubbe SCC, Guerra CF (2018) J Phys Chem A 122:1813
Domagała M, Simon S, Palusiak M (2022) Int J Mol Sci 23:233
Mahanta S, Paul BK, Singh RB, Guchhait N (2010) J Comput Chem 32:1
Espinosa E, Lecomte C, Molinsa E (1999) Chem Phys Lett 300:745
Emamian S, Lu T, Kruse H, Emamian H (2019) J Comput Chem 40:28681
Johnson ER, Keinan S, Mori-Sanchez P, Contreras-Garcia J, Cohen AJ, Yang W (2010) J Am Chem Soc 132:6498
Bader RFW, Essen H (1984) J Chem Phys 80:1943
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, Revision A 02-SMP. Gaussian Inc, Wallingford
Foresman JB, Frisch Æ (1996) Exploring Chemistry with Electronic Structure Methods, 2nd edn. Gaussian Inc, Pittsbugh
Silva TC, dos Santos PM, Castrode AA, Rocha MVJ, Ramalho TC (2018) Theor Chem Acc 137:146
Bayat A, Fattahi A (2018) J Phys Org Chem 32:e3919
Vydrov OA, Scuseria GE (2006) J Chem Phys 125:234109
Perdew JP, Burke K, Ernzerhof M (1996) Phys Rev Lett 77:3865
Zhao Y, Truhlar DG (2008) Theor Chem Acc 120:215
Mota AJ, Neuhold J, Drescher M, Lemouzy S, González L, Maulide N (2017) Org Biomol Chem 15:7572
Biegler-König F, Schönbohm J, Bayles D (2001) J Comput Chem 22:24
Lu T, Chen F (2012) J Comput Chem 33:580
Lu T, Chen F (2012) J Mol Graph Model 38:314
Humphrey W, Dalke A, Schulten K (1996) J Mol Graph 14:33
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Paul, B.K., Rakshit, P. A computational assessment of the O−H⋅⋅⋅O intramolecular hydrogen-bonding in substituted phenalenes: diverse degrees of covalence. Monatsh Chem 154, 605–613 (2023). https://doi.org/10.1007/s00706-023-03070-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-023-03070-7