Abstract
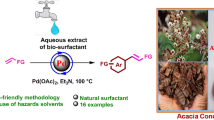
In the present work, we have performed eco-benign strategy of in situ formation of PdNPs using aqueous extract of Acacia auriculiformis pods for Mizoroki–Heck coupling. A series of (E)-1-(3-argioallyl)indoline-2,3-diones have been synthesized from coupling of aryl halides with allyl isatins. The PdNPs were characterized by transmission electron microscopy (TEM) which revealed PdNPs size of around 6 nm. The key features of the present method are synthesis of novel derivatives of (E)-1-(3-argioallyl)indoline-2,3-diones, PdNPs in aqueous biosurfactant extract as catalytic system which can be recycled for four times without significant loss in the catalytic activity, no need of external ligand. The influence of various parameters such as the nature and amount of base, source of Pd, screening of available surfactants as well as the effect of temperature has been investigated.
Graphical abstract






Similar content being viewed by others
References
Whitcombe NJ, HiiK KM, Gibson SE (2001) Tetrahedron 57:7449
Farina V (2004) Adv Synth Catal 346:1553
Hassan J, Sevignon M, Gozzi C, Schulz E, Lemaire M (2002) Chem Rev 102:1359
Albaneze Walker J, Murry JA, Soheili A, Ceglia S, Springfield SA, Bazaral C, Dormer PG, Hughes DL (2005) Tetrahedron 61:6330
Kertesz M, Choi CH, Yang S (2005) Chem Rev 105:3448
Simon MO, Li CJ (2012) Chem Soc Rev 41:1415
Anastas P, Eghbali N (2010) Chem Soc Rev 39:301
Carril M, Martin RS, Dominguez E (2008) Chem Soc Rev 37:639
Shaughnessy KH (2006) Eur J Org Chem 2006:1817
DeSimone JM (2002) Science 297:799
Savage PE (1999) Chem Rev 99:603
Gawande MB, Bonifacio VD, Luque R, Branco PS, Varma RS (2013) Chem Soc Rev 42:5522
Nasrollahzadeh M, Sajadi SM, Maham M (2015) J Catal A 396:297
LaSorella G, Strukul G, Scarso A (2015) Green Chem 17:644
Khazaei A, Rahmati S, Hekmatian Z, Saeednia S (2013) J Mol Catal A Chem 372:160
Puthiaraja P, Pitchumani K (2014) Green Chem 16:4223
Phillip J, Anna T, Victor S, Valerie JP (2021) Adv Colloid Interface Sci 288:102340
Chahdoura F, Favier I, Pradel C, Mallet-Ladeira S, Gómez M (2015) Catal Commun 63:47
Leonie AS, Maria GCS, Italo JBD, Karen GOB, Beatriz GR, Ivison AS, Matthew ST, Ibrahim MB (2022) Biochem Eng J 181:108377
Markandea AR, Patelb D, Varjanic S (2021) Bioresour Technol 330:124963
Patil SP, Jadhav SN, Rode CV, Shejwal RV, Kumbhar AS (2020) Transit Met Chem 45:403
Han K, Zhou Y, Liu F, Guo Q, Wang P, Yang Y, Song B, Liu W, Yao Q, Teng Y, Yu P (2014) Bioorg Med Chem Lett 24:591
Tarek AF, Bin-Jubair FAS (2010) Int J Res Pharm Sci 1:113
Matheus ME, Violante FA, Garden SJ, Pinto AC, Fernandes PD (2007) Eur J Pharmacol 556:200
Chohan ZH, Pervez H, Rauf A, Khan KM, Supuran CT (2004) J Enzyme Inhib Med Chem 19:417
Kilpin KJ, Henderson W, Nicholson BK (2007) Polyhedron 26:204
Verma M, Pandeya SN, Singh KN, Stables JP (2004) Acta Pharm 54:49
Rasmussen HB, MacLeod JK (1997) J Nat Prod 60:1152
Khokhar S, Feng Y, Campitelli MR, Quinn RJ, Hooper JNA, Ekins MG, Davis RA (2013) J Nat Prod 76:2100
Hughes CC, Fenical W (2010) J Am Chem Soc 132:2528
Asati N, Yadava RN (2014) Int J Pharm Res Bio-Sci 3:341
Mandal P, Sinha Babu SP, Mandal NC (2005) Fitoterapia 76:462
Shmidt MS, Reverdito AM, Kremenchuzky L, Perillo IA, Blanco MM (2008) Molecules 13:831
Shrestha R, Lee GJ, Lee YR (2016) RSC Adv 6:63782
Sele AM, Bremner JB, Willis AC, Haritakun R, Griffith R, Keller PA (2015) Tetrahedron 43:8357
Jha M, Shelke GM, Kumar A (2014) Eur J Org Chem 16:3334
Vaidya GN, Nagpure M, Kumar D (2021) ACS Sustain Chem Eng 9:1846
Jha M, Shelke GM, Kumar A (2014) Eur J Org Chem 2014:3334
Trost BM, Kalnmals CA, Ramakrishnan D, Ryan MC, Smaha RW, Parkin S (2020) Org Lett 22:2584
Acknowledgements
One of the authors, PMM is grateful to the Council of Scientific and Industrial Research (CSIR), New Delhi, Government of India, for the award of the Junior Research Fellowship (File no. 09/ 816(0040)/2017-EMR-I).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Patil, M.V., Mhaldar, P.M. & Pore, D.M. Mizoroki–Heck coupling: a novel approach for synthesis of (E)-1-(3-argioallyl)indoline-2,3-dione. Monatsh Chem 153, 1243–1250 (2022). https://doi.org/10.1007/s00706-022-02978-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-022-02978-w