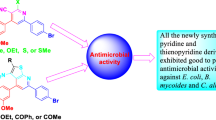
Abstract
A new series of pyrazolyl–chalcone derivatives was prepared in moderate yields via Claisen–Schmidt condensation reaction of 4-acetylpyrazole derivatives with the corresponding aldehydes. The newly synthesized compounds have been fully characterized by 1H NMR, 13C NMR, IR, mass spectrometry, and elemental analysis. The in vitro antimicrobial and anti-cancer activities of the novel compounds were evaluated. Depending on the structure of the molecule, different types of compounds have varying effects on microbial growth effectiveness. 3-(2,4-Dimethoxyphenyl)-1-[3,5-dimethyl-1-(4-nitrophenyl)-1H-pyrazol-4-yl]prop-2-en-1-one gave the highest antibacterial activity (20 mm) against B. mycoides, whereas 3-(4-chlorophenyl)-1-[3,5-dimethyl-1-(4-nitrophenyl)-1H-pyrazol-4-yl]prop-2-en-1-one and 1-[3,5-dimethyl-1-(4-nitrophenyl)-1H-pyrazol-4-yl]-3-(p-tolyl)prop-2-en-1-one had equivalent antibacterial activity (17 mm) against E. coli. The 4-chlorophenyl derivative exhibited the most potent antifungal activity against C. albicans (17 mm). The anti-cancer activity of the prepared compounds was tested against four human cancer cell lines namely A549 (lung carcinoma), MCF7 (human caucasian breast adenocarcinoma), HePG2 (human hepatocellular carcinoma cell line), and BJ1 (normal skin fibroblast). 1-[3,5-Dimethyl-1-(4-nitrophenyl)-1H-pyrazol-4-yl]-3-(p-tolyl)prop-2-en-1-one emerged as the most promising compound with IC50 = 44.3 µg/cm3 against A549 and IC50 = 57.9 µg/cm3 against HePG2. Its gene expression, DNA damage values, and DNA fragmentation percentages have been discussed. The expression values of ISL1 and MALL, ASNS and ACLY genes were decreased significantly in treated lung and liver cell lines respectively and positive control compared with negative samples. The expression levels of ISL1 and MALL genes were downregulated in positive control lung cell lines much lower than those in the p-tolyl substituted derivative. The expression levels of ASNS and ACLY genes were downregulated similar to those in positive control liver cell lines. The DNA damage values and DNA fragmentation percentages were increased significantly (P < 0.01) in the treated lung and liver sample compared with the negative control.
Graphical abstract




Similar content being viewed by others
References
Bandgar BP, Gawande SS, Bodade RG, Gawande NM, Khobragade CN (2009) Bioorg Med Chem 17:8168
Bekhit AA, Abdel-Aziem T (2004) Bioorg Med Chem 12:1935
Hsieh H-K, Tsao L-T, Wang J-P, Lin C-N (2000) J Pharm Pharmacol 52:163
Onyilagha JC, Malhotra B, Elder M, French CJ, Towers GHN (1997) Can J Plant Pathol 19:133
Lin C-N, Hsieh H-K, Ko H-H, Hsu M-F, Lin H-C, Chang Y-L, Chung M-I, Kang J-J, Wang J-P, Teng C-M (2001) Drug Dev Res 53:9
Asiri AM, Khan SA (2011) Molecules 16:523
Li R, Kenyon GL, Cohen FE, Chen X, Gong B, Dominguez JN, Davidson E, Kurzban G, Miller RE, Nuzum EO, Rosenthal PJ, McKerrow JH (2002) J Med Chem 38:5031
Heidari MR, Foroumadi A, Amirabadi A, Samzadeh-Kermani A, Azimzadeh BS, Eskandarizadeh A (2009) Ann NY Acad Sci 1171:399
Shenvi S, Kumar K, Hatti KS, Rijesh K, Diwakar L, Reddy GC (2013) Eur J Med Chem 62:435
Sashidhara KV, Kumar A, Kumar M, Sarkar J, Sinha S (2010) Bioorg Med Chem Lett 20:7205
Fathi EM, Sroor FM, Mahrous KF, Mohamed MF, Mahmoud K, Emara M, Elwahy AHM, Abdelhamid IA (2021) Chem Select 6:6202
Mohamed MF, Sroor FM, Ibrahim NS, Salem GS, El-Sayed HH, Mahmoud MM, Wagdy M-AM, Ahmed AM, Mahmoud A-AT, Ibrahim SS, Ismail MM, Eldin SM, Saleh FM, Hassaneen HM, Abdelhamid IA (2020) Invest New Drugs 39:98
Karrouchi K, Radi S, Ramli Y, Taoufik J, Mabkhot Y, Al-aizari F, Ansar MH (2018) Molecules 23:134
Khan MF, Alam MM, Verma G, Akhtar W, Akhter M, Shaquiquzzaman M (2016) Eur J Med Chem 120:170
Iglesias-Arteaga MA, Pérez-Fernández R, Goya P, Elguero J (2014) ARKIVOC 2014:233
Fustero S, Sánchez-Roselló M, Barrio P, Simón-Fuentes A (2011) Chem Rev 111:6984
Fustero S, Simón-Fuentes A, Sanz-Cervera JF (2009) Org Prep Proced Int 41:253
Gomha SM, Edrees MM, Faty RAM, Muhammad ZA, Mabkhot YN (2017) Chem Cent J 11:37
Altalbawy F (2013) Int J Mol Sci 14:2967
Zagni C, Citarella A, Oussama M, Rescifina A, Maugeri A, Navarra M, Scala A, Piperno A, Micale N (2019) Int J Mol Sci 20:945
Bennani FE, Doudach L, Cherrah Y, Ramli Y, Karrouchi K, Ansar Mh, Faouzi MEA (2020) Bioorg Chem 97:103470
Othman IMM, Alamshany ZM, Tashkandi NY, Gad-Elkareem MAM, Anwar MM, Nossier ES (2021) Bioorg Chem 114:105078
Ansari A, Ali A, Asif M, Shamsuzzaman S (2017) New J Chem 41:16
Surendra Kumar R, Arif IA, Ahamed A, Idhayadhulla A (2016) Saudi J Biol Sci 23:614
Jakob KS, Ganguly S (2016) Int J Pharm Pharm Sci 8:75
Alam R, Wahi D, Singh R, Sinha D, Tandon V, Grover A, Rahisuddin (2016) Bioorg Chem 69:77
Bekhit AA, Hassan AMM, Abd El Eazik HA, El-Miligy MMM, El-Agroudy EJ, Bekhit AE-DA (2015) Eur J Med Chem 94:30
Karcı F, Karcı F, Demirçalı A, Yamaç M (2013) J Mol Liq 187:302
Burschka J, Kessler F, Nazeeruddin MK, Grätzel M (2013) Chem Mater 25:2986
Chou P-T, Chi Y (2007) Chem Eur J 13:380
Kauhanka UM, Kauhanka MM (2006) Liq Cryst 33:121
Wang M, Zhang J, Liu J, Xu C, Ju H (2002) J Lumin 99:79
Tantawy MA, Sroor FM, Mohamed MF, El-Naggar ME, Saleh FM, Hassaneen HM, Abdelhamid IA (2020) Anti-Cancer Agents Med Chem 20:70
Abdelhamid IA, Abdelmoniem AM, Sroor FM, Ramadan MA, Ghozlan SAS (2020) Synlett 31:895
Sroor FM, Basyouni WM, Tohamy WM, Abdelhafez TH, El-awady MK (2019) Tetrahedron 75:130749
Shawali AS, Hassaneen, HM (1976) Indian J Chem 7: 549
Stanovnik B, Svete J (2002) Product class 1: pyrazoles. In: Neier R, Bellus D (eds) Science of synthesis 2: hetarenes, vol 12. Thieme, Stuttgart, New York
Abouelnaga AM, Meaz TM, Othman AM, Ghazy RA, El Nahrawy AM (2020) SILICON 13:623
Mohamed SAA, El-Sakhawy M, Nashy ELSHA, Othman AM (2019) Int J Biol Macromol 136:774
Sroor FM, Othman AM, Tantawy MA, Mahrous KF, El-Naggar ME (2021) Bioorg Chem 112:104953
Thabrew MI, Hughes RD, McFarlane IG (1997) J Pharm Pharmacol 49:1132
Watanabe T, Miura T, Degawa Y, Fujita Y, Inoue M, Kawaguchi M, Furihata C (2010) Cancer Cell Int 10:2
Saur D, Seidler B, Schneider G, Algül H, Beck R, Senekowitsch-Schmidtke R, Schwaiger M, Schmid RM (2005) Gastroenterology 129:1237
Yang Q, Feng MH, Ma X, Li HC, Xie W (2017) Oncol Lett 14:6071
Olive PL, Banáth JP, Durand RE (2012) Radiat Res 178:AV35
Collins A, Dusinska M, Franklin M, Somorovska M, Petrovska H, Duthie S, Fillion L, Panayiotidis M, Raslova K, Vaughan N (1997) Environ Mol Mutagen 30:139
Yawata A, Adachi M, Okuda H, Naishiro Y, Takamura T, Hareyama M, Takayama S, Reed JC, Imai K (1998) Oncogene 16:2681
Hegarty AF, Cashman MP, Scott FL (1972) J Chem Soc Perkin Trans 2:1381
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kamel, M.G., Sroor, F.M., Othman, A.M. et al. Structure-based design of novel pyrazolyl–chalcones as anti-cancer and antimicrobial agents: synthesis and in vitro studies. Monatsh Chem 153, 211–221 (2022). https://doi.org/10.1007/s00706-021-02886-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-021-02886-5