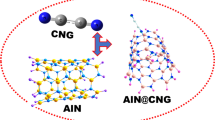
Abstract
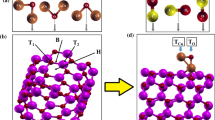
The adsorption of N2, O2, H2O, hydrogen chloride (HCl), Cl2, hypochlorous acid (HClO), and ClO2 gases was explored onto an AlN nanotube (AlNNT) through density functional theory computations. As N2, O2, H2O, HCl, Cl2, and HClO approach the AlNNT, their adsorption releases 7.1, 12.6, 22.3, 26.5, 30.2, and 41.2 kJ/mol of energy, respectively, indicating a physisorption. In addition, the electronic properties of the nanotube do not change significantly. As chlorine dioxide (ClO2) approaches the AlNNT, its adsorption releases 97.4 kJ/mol of energy. Electronic analysis showed that the AlNNT HOMO–LUMO gap reduces from 4.10 to 2.80 eV (~ − 31.7%) by ClO2 adsorption and the electrical conductivity increases significantly. Therefore, the AlNNT can generate electrical signals when the ClO2 molecules approach, being a hopeful sensor. It was found that this nanotube can selectively detect ClO2 gas among the mentioned molecules. The recovery time for the AlNNT was computed to be 8.0 s for ClO2 desorption, representing a short recovery time.
Graphical abstract












Similar content being viewed by others
References
Gharibzadeh F, Vessally E, Edjlali L, Es’haghi M, Mohammadi R (2020) Iran J Chem Chem Eng 39:51–62. https://doi.org/10.30492/ijcce.2020.106867.3568
Vessally E, Babazadeh M, Alipour F, Hosseinian A, Kheirollahi Nezhad PD (2021) Iran J Chem Chem Eng 40:691–703. https://doi.org/10.30492/ijcce.2020.122123.3987
Ahmadi S, Hosseinian A, Kheirollahi Nezhad PD, Monfared A, Vessally E (2019) Iran J Chem Chem Eng 38:1–19. https://doi.org/10.30492/ijcce.2019.33786
Vessally E, Mohammadi S, Abdoli M, Hosseinian A, Ojaghloo P (2020) Iran J Chem Chem Eng 39:11–19. https://doi.org/10.30492/ijcce.2019.36288
Vessally E, Farajzadeh P, Najafi E (2021) Iran J Chem Chem Eng 40:1001–1011. https://doi.org/10.30492/ijcce.2021.141635.4498
Ma X, Kexin Z, Yonggang W, Ebadi AG, Toughani M (2021) Iran J Chem Chem Eng. https://doi.org/10.30492/IJCCE.2021.529010.4694
Hashemzadeh B, Edjlali L, Kheirollahi Nezhad PD, Vessally E (2021) Chem Rev Lett. https://doi.org/10.22034/crl.2020.187273.1087
Salehi N, Vessally E, Edjlali L, Alkorta I, Eshaghi M (2000) Chem Rev Lett 3:207–217. https://doi.org/10.22034/crl.2020.230543.1056
Sreerama L, Vessally E, Behmagham F (2020) J Chem Lett 1:9–18. https://doi.org/10.22034/jchemlett.2020.106645
Kumar S (2008) ALD growth of a novel mixed-phase barrier for seedless copper electroplating applications. State University of New York, Albany
Majedi S, Sreerama L, Vessally E, Behmagham F (2020) J Chem Lett 1:25–31. https://doi.org/10.22034/jchemlett.2020.107760
Moradi O, Zare K, Monajjemi M, Yari M, Aghaie H (2010) Fullerenes Nanotub Carbon Nanostruct 18:285
Moradi O, Zare K (2011) Fullerenes Nanotub Carbon Nanostruct 19:628
Ahmadi A, Kamfiroozi M, Beheshtian J, Hadipour NL (2011) Struct Chem 22:1261–1265
Baei MT, Peyghan AA, Bagheri Z (2012) Chin Chem Lett 23:965–968
Baei MT, Peyghan AA, Bagheri, Z (2013) Struct Chem 24:1099–1103
Beheshtian J, Peyghan AA, Bagheri Z, Tabar MB (2013) Struct Chem 25:1–7
Moradi M, Peyghan AA, Bagheri Z, Kamfiroozi M (2012) J Mol Model 18:3535–3540
Wang Z, Lei Q, Wang Z, Yuan H, Cao L, Qin N, Liu J (2020) Chem Eng J 395:125180. Lausanne, Switzerland. https://doi.org/10.1016/j.cej.2020.125180
Peyghan AA, Baei MT, Moghimi M, Hashemian S (2012) Comput Theor Chem 997:63–69
Koao LF, Hone FG, Dejene FB (2020) J Nanostruct Chem 10:1
Aragaw BA (2020) J Nanostruct Chem 10:9
Malinga NN, Jarvis ALL (2020) J Nanostruct Chem 10:55
Peyghan AA, Noei M (2014) Physica B: Condensed Matter 432:105–110
Peyghan AA, Soleymanabadi H (2015) Current Science 108:1910–1914
Robati D, Bagheriyan S, Rajabi M, Moradi O, Peyghan AA (2016) Physica E 83:1–6
Zhang X, Tang Y, Zhang F, Lee C (2016) Adv Energ Mater 6(11):1502588. https://doi.org/10.1002/aenm.201502588
Ji B, Zhang F, Song X, Tang Y (2017) Adv Mater (Weinheim) 29(19):1700519. https://doi.org/10.1002/adma.201700519
Beheshtian J, Baei MT, Peyghan AA, Bagheri Z (2013) J Mol Model 19:943
He C, Wang J, Fu L, Zhao C, Huo J (2021) Chin Chem Lett. https://doi.org/10.1016/j.cclet.2021.09.009
Jiang L, Wang Y, Wang X, Ning F, Wen S, Zhou Y, Zhou F (2021) Appl Sci Manuf 147:106461. https://doi.org/10.1016/j.compositesa.2021.106461
Hu L, Huang X, Zhang S, Chen X, Dong X, Jin H, Jiang Z (2021) Mater Electron 32(19):23728. https://doi.org/10.1007/s10854-021-06464-7
Li Y, Macdonald DD, Yang J, Qiu J, Wang S (2020) Corros Sci 163:108280. https://doi.org/10.1016/j.corsci.2019.108280
Xie Y, Meng X, Mao D, Qin Z, Wan L, Huang Y (2021) ACS Appl Mater Interfaces 13(27):32161–32174. https://doi.org/10.1021/acsami.1c07148
Rezaei A, Ghiasi R, Marjani A (2020) J Nanostruct Chem 10:179
Najafi F (2020) J Nanostruct Chem 10:227
Foroutan M, Fatemi SJ, Fatemi SM (2020) J Nanostruct Chem 10:265
Rodrigues BS, Almeida VA, Claudino CH, Ponce-de-Leon C, Bavykin DV, Souza JS (2020) J Nanostruct Chem 10:363
Baei MT (2012) Monatsh Chem 143:545
Mirzaei M, Mirzaei M (2011) Monatsh Chem 142:115
Noei M, Salari AA, Ahmadaghaei N, Bagheri Z, Peyghan AA (2013) C R Chim 16:985
Samadizadeh M, Rastegar SF, Peyghan AA (2015) Phys E 69:75
Mohammadi R, Hosseinian A, Khosroshahi ES, Edjlali L, Vessally E (2018) Phys E 98:53
Baei MT, Peyghan AA, Bagheri Z (2013) Superlattices Microstruct 53:9
Hu M, Wang Y, Yan Z, Zhao G, Zhao Y, Xia L, Zhuang X (2021) Mater Energ Sustain 9(24):14093–14100. https://doi.org/10.1039/D1TA01505B
Ahmadi A, Hadipour NL, Kamifiroozi M, Bagheri Z (2012) Sens Actuators B 161:1025
Ahmadi Peyghan A, Omidvar A, Hadipour NL, Bagheri Z, Kamfiroozi M (2012) Phys E 44:1357
Fu Y, Chen H, Guo R, Huang Y, Toroghinejad MR (2021) J Alloy Compd 888:161507. https://doi.org/10.1016/j.jallcom.2021.161507
Yang M, Kong Q, Feng W, Yao W, Wang Q (2021) Appl Surf Sci 569:150984. https://doi.org/10.1016/j.apsusc.2021.150984
Zhang C, Liu X, Liu C, Luo X (2021) J Kansas Entomol Soc. https://doi.org/10.2317/0022-8567-93.4.267
Yan Y, Feng L, Shi M, Cui C, Liu Y (2020) Food Chem 306:125589. https://doi.org/10.1016/j.foodchem.2019.125589
Shi M, Wang F, Lan P, Zhang Y, Zhang M, Yan Y, Liu Y (2021) Food Sci Technol 138:110677. https://doi.org/10.1016/j.lwt.2020.110677
Baei MT, Peyghan AA, Bagheri Z, Tabar MB (2012) Phys Lett A 377:107
Priya DD, Khan MMR, Roopan SM (2020) J Nanostruct Chem 10:289
Rita A, Sivakumar A, Sahaya Jude-Dhas S, Martin-Britto-Dhas SA (2020) J Nanostruct Chem 10:309
González-Ballesteros N, Rodríguez-Argüelles MC, Lastra-Valdor M, González-Mediero G, Rey-Cao S, Grimaldi M, Cavazza A, Bigi F (2020) J Nanostruct Chem 10:317
Kozlovskiy A, Zdorovets M, Kenzhina I, Berguzinov A, Tishkevich D, Zubar T, Trukhanov A (2020) J Nanostruct Chem 10:331
Rehman Y, Copet C, Morlando A, Huang XF, Konstantinov K (2020) J Nanostruct Chem 10:347
Moradi O, Zare K (2013) Fullerenes Nanotub Carbon Nanostruct 21:449
Adhikari K, Ray A (2011) Phys Lett A 375:1817
Zhao G, Li X, Huang M, Zhen Z, Zhong Y, Chen Q, Zhao X, He Y, Hu R, Yang T (2017) Chem Soc Rev 46:4417
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rahmani, Z., Fosshat, S., Alizadeh, S.M.S. et al. A theoretical survey on the chlorine dioxide (ClO2) and its decomposed species detection by the AlN nanotube in presence of environmental gases. Monatsh Chem 153, 21–29 (2022). https://doi.org/10.1007/s00706-021-02873-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-021-02873-w