Abstract
An expedient three-component reaction for the synthesis of acenaphtho[1',2':4,5]pyrrolo[2,3-d]pyrimidine, (hydropyrimidinyl) acenaphtho[1',2':4,5]pyrrolo[2,3-d]pyrimidine, and phenanthro[9,10-b]pyrimido[4,5-e][1,4]oxazine is described. The desired products were obtained in good to high yields. This work has the advantages of convenient operation, mild reaction conditions, simple workup, and provides an entry point to fused heterocyclic structures.
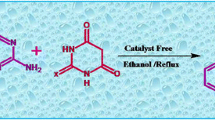
Graphic abstract


Similar content being viewed by others
References
Liu YY, Yu XY, Chen JR, Qiao MM, Qi X, Shi DQ, Xiao WJ (2017) Angew Chem Int Ed 56:9527
Sarkar R, Mukhopadhyay C (2014) Tetrahedron Lett 55:2618
Zhu J, Bienayme H (2005) Multicomponent reactions. Wiley-VCH, Weinheim
Singh MS, Chowdhury S (2012) RSC Adv 2:4547
Chen Z, Wu J (2010) Org Lett 12:4856
Pando O, Stark S, Dnkert A, Porzel A, Preusentanz R, Wessjohann LA (2011) J Am Chem Soc 133:7692
Dömling A (2006) Chem Rev 106:17
Ramon DJ, Yus M (2005) Angew Chem Int Ed 44:1602
Yavari I, Khajeh Khezri A (2018) Synthesis 50:3947
Yavari I, Khajeh Khezri A, Halvagar MR (2018) Synthesis 29:2011
Hall DG, Rybak T, Verdelet T (2016) Acc Chem Res 49:2489
Mao ZY, Liu YW, Han P, Dong HQ, Si CM, Wei BG, Lin GQ (2018) Org Lett 20:1090
Li C, Zhang F (2018) ChemistrySelect 3:1815
Wang X, Li M, Yang Y, Guo M, Tang X, Wang G (2018) Adv Synth Catal 360:2151
Liu Z, Zhang L, Sun J, Yang C (2014) Chin J Chem 32:172
Sundberg RJ (1977) J Heterocycl Chem 14:517
Katritzky A, Rees CW, Scriven EFV (eds) (1996) Comprehensive heterocyclic chemistry II, vol 2. Pergamon Press, Oxford, p 119
Matano Y, Imahori H (2009) Acc Chem Res 42:1193
Higgins S (1997) Chem Soc Rev 26:247
Penning TM (1985) J Pharm Sci 74:651
Bal C, Baldeyrou B, Moz F, Lansiaux A, Colson P, Kraus-Berthier L, Leonce S, Pierre A, Boussard BM, Rousseau A, Wierzbicki M, Bailly C (2004) Biochem Pharmacol 68:1911
Brown DW, Graupner PR, Sainsbury M, Shertzer HG (1991) Tetrahedron 47:4383
Butera JA, Antane SA, Hirth B, Lennox JR, Sheldon JH, Norton NW, Warga D, Argentieri TM (2001) Bioorg Med Chem Lett 11:2093
Pradhan K, Paul S, Das AR (2015) RSC Adv 5:12062
Ramos LM, Rodrigues MO, Neto BAD (2019) Org Biomol Chem 17:7260
Acknowledgements
We gratefully acknowledge the financial support of this research from Imam Khomeini International University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Amiri, Z., Bayat, M. Synthesis of acenaphtho[1',2':4,5]pyrrolo[2,3-d]pyrimidine derivatives via one-pot three-component reaction. Monatsh Chem 152, 1291–1296 (2021). https://doi.org/10.1007/s00706-021-02836-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-021-02836-1