Abstract
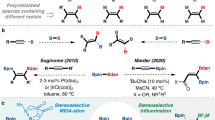
A practical synthetic method for the synthesis of aryl bromide was developed through regioselective bromination of boronic acid in the presence of poly-N-bromosulfonamide-melamine (PBBSM). In this regard, a novel heterogeneous support, cross-linked poly sulfonamide-melamine, has been successfully synthesized to stabilize bromine with high surface functional group density (6.6 mmol Br+/g). The prepared reagent is a novel brominating reagent that combines the effective functions of N-bromosulfonamide, N-bromosulfonamide-melamine, and melamine groups. The structure of PBBSM was characterized using XRD, FT–IR, 1H NMR, TGA, FE-SEM, EDX, and TGA analysis.
Graphic abstract







Similar content being viewed by others
References
Wilcken R, Zimmermann MO, Lange A, Joerger AC, Boeckler FM (2013) J Med Chem 56:1363
Christophersen C (1985) Acta Chem Scand B 39:515
Mamaghani M, Sheykhan M, Sadeghpour M, Tavakoli F (2018) Monatsh Chem 149:1437
de la Mare PBD (1976) Electrophilic halogenation. Cambridge University Press, Cambridge
March J (2000) Advance organic chemistry, 4th edn. Wiley, New York
Barhate NB, Gajare AS, Wakharkar RD, Bedekar AV (1998) Tetrahedron Lett 39:6349
Auerbach J, Weissman S, Blacklock ATJ, Angeles MR, Hoogsteen K (1993) Tetrahedron Lett 34:931
Dundik S, Fu GC (2012) J Am Chem Soc 134:10693
Hall D (ed) (2011) Boronic acids: preparation and applications in organic synthesis, medicine and materials, 2nd edn. Wiley, Weinheim, p 1
Tao CZ, Cui X, Liu AX, Liu L, Guo QX (2007) Tetrahedron Lett 48:3525
Mohammed S, Padala AK, Dar A, Singh B, Sreedhar B, Vishwakarma RA, Bharate SB (2012) Tetrahedron 68:8156
Kar A, Sayeed IA, Low WF, Kaiser HM, Beller M, Tse MK (2007) Org Lett 9:3405
Qi HL, Chen DS, Ye JS, Huang JM (2013) J Org Chem 78:7482
Chen DS, Huang JM (2013) Synlett 24:499
Prakash GKS, Chacko S, Panja C, Thomas TE, Gurung L, Rasul G, Mathew T, Olah GA (2009) Adv Synth Catal 351:1567
Zarei M, Noroozizadeh E, Moosavi-Zare AR, Zolfigol MA (2018) J Org Chem 83:3645
Partridge BM, Hartwig JF (2013) Org Lett 15:140
Molander GA, Cavalcanti LN (2011) J Org Chem 76:7195
Furuya T, Kaiser HM, Ritter T (2008) Angew Chem Int Ed 47:5993
Furuya T, Benitez D, Tkatchouk E, Strom AE, Tang PP, Goddard WA, Ritter T (2010) J Am Chem Soc 132:3793
Furuya T, Ritter T (2009) Org Lett 11:2860
Kabalka GW, Gooch EE (1980) J Org Chem 45:3578
Kabalka GW, Gooch EE (1981) J Org Chem 46:2582
Kabalka GW, Mereddy AR (2004) Organometallics 23:4519
Kabalka GW, Sastry KAR, Sastry U, Somayaji V (1982) Org Prep Proc Int 1:359
Thiebes C, Prakash GKS, Petasis NA, Olah GA (1998) Synlett 2:141
Szumigala RH Jr, Devine PN, Gauthier DR Jr, Volante RP (2004) J Org Chem 69:566
Zhang GY, Lv GL, Li LP, Chen F, Cheng J (2011) Tetrahedron Lett 52:1993
Fu F, Gurung L, Czaun M, Mathew T, Prakash GKS (2019) Tetrahedron Lett 60:151020
Fu Z, Hao G, Fu Y, He D, Tuo X, Guo S, Cai H (2020) Org Chem Front 7:590
Goljani H, Tavakkoli Z, Sadatnabi A, Nematollahi D (2020) Org Lett 22:5920
Li B, Gong R, Luo Y, Tan B (2011) Soft Matter 7:10910
Alavinia S, Ghorbani-Vaghei R (2020) New J Chem 44:13062
Alavinia S, Ghorbani-Vaghei R, Rakhtshah J, Seyf JY, Arabian IA (2020) Appl Organomet Chem 34:e5449
Alavinia S, Ghorbani-Vaghei R (2020) J Phys Chem Solids 146:109573
Hamidi Dastjerdi F, Ghorbani-Vaghei R, Alavinia S (2020) Catal Lett 150:3514
Solgi S, Ghorbani-Vaghei R, Alavinia S (2021) J Porous Mater 28:289
Zafari S, Ghorbani-Vaghei R, Alavinia S (2021) Mater Chem Phys 270:124840
Ghorbani-Vaghei R, Jalili H (2005) Synthesis 7:1099
Ghorbani-Vaghei R, Shahbazi H, Veisi H (2012) Tetrahedron Lett 53:2325
Ghorbani-Vaghei R, Chegini M, Veisi H, Karimi-Tabar M (2009) Tetrahedron Lett 50:1861
Veisi H, Ghorbani-Vaghei R (2010) ChemInform 66:7445
Amornpitoksuk P, Suwanboon S, Sirimahachai U, Randorn C, Yaemsunthorn K (2016) Bull Mater Sci 39:1507
Jia L, Wang D, Liu L, Zhang S, Xu T (2014) Des Monomers Polym 17:425
He W, Zhanga R, Cai M (2017) RSC Adv 7:764
Wu H, Hynes J Jr (2010) Org Lett 12:1192
Acknowledgements
We are thankful to Bu-Ali Sina University, Center of Excellence in Development of Environmentally Friendly Methods for Chemical Synthesis (CEDEFMCS), for financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Alavinia, S., Ghorbani-Vaghei, R. Poly-N-bromosulfonamide-melamine as a novel brominating reagent for regioselective ipso-bromination of arylboronic acids. Monatsh Chem 152, 1269–1276 (2021). https://doi.org/10.1007/s00706-021-02827-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-021-02827-2