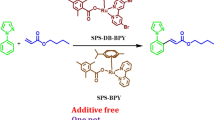
Abstract
New triazolium Schiff bases (TSBs) were synthesised via a simple and high throughput process. The new salts were successfully characterised. When reacted with Cu(CH3CN)4PF6, the TSB salts formed mononuclear triazole Schiff base copper(I) complexes and dinuclear complexes that were also characterised. The copper complexes were generated in situ (mixtures of TSB salts with Cu(CH3CN)4PF6) and applied as homogeneous catalysts for the C–C coupling of a variety of aryl ketones with aryl alcohols, from which significant reactivity was observed. Reaction conditions were optimised, and the results indicate that the catalyst systems are very robust. A catalyst concentration of 10 mol% efficiently and selectively catalysed the α-alkylation of methyl phenyl ketone and its derivatives to afford up to 94% yield of 1,3-diphenylpropan-1-one and its analogues. The process is adaptable with analogues of acetophenone and benzyl alcohol bearing various regulating substituents tolerated.
Graphic abstract



Similar content being viewed by others
References
Trost BM, Fleming I (1991). In: Schreiber SL (ed) Selectivity, strategy and efficiency in modem organic chemistry. Pergamon Press, Oxford
Obora Y (2014) ACS Catal 4:3972
Shen D, Poole DL, Shotton CC, Kornahrens AF, Healy MP, Donohoe TJ (2015) Angew Chem Int Ed 54:1642
Rueping M, Phapale VB (2012) Green Chem 14:55
Taguchi K, Nakagawa H, Hirabayashi T, Sakaguchi S, Ishii Y (2004) J Am Chem Soc 126:72
Yan F, Zhang M, Wang X, Xie F, Chen M, Jiang H (2014) Tetrahedron 70:1193
Cho CS, Kim BT, Kim T, Shim SC (2002) Tetrahedron Lett 43:7987
Kovalenko, OO, Lundberg H, Hübner D, Adolfsson H (2014) Eur J Org Chem 6639. https://doi.org/10.1002/ejoc.201403032
Kuwahara T, Fukuyama T, Ryu I (2012) Org Lett 14:4703
Martınez R, Brand GJ, Ramon DJ, Yus M (2005) Tetrahedron Lett 46:3683
Martınez R, Ramon DJ, Yus M (2006) Tetrahedron 62:8988
Chan LK, Poole DL, Shen D, Healy MP, Donohoe TJ (2014) Angew Chem Int Ed 53:761
Cho CS, Seok HJ, Shim SC (2005) J Heterocycl Chem 42:1219
Satyanarayana P, Reddy GM, Maheswaran H, Kantam ML (2013) Adv Synth Catal 355:1859
Wang R, Huang L, Du Z, Feng H (2017) J Organomet Chem 846:40
Elangovan S, Sortais JB, Beller M, Darcel C (2015) Angew Chem Int Ed 54:14483
Pan X, Li M, Gu Y (2014) Chem Asian J 9:268
Peria-Lopez M, Piehl P, Elangovan S, Neumann H, Beller M (2016) Angew Chem Int Ed 55:14967
Zhang G, Wu J, Zeng H, Zhang S, Yin Z, Zheng S (2017) Org Lett 19:1080
Dixit M, Mishra M, Joshi PA, Shah DO (2013) Catal Commun 33:80
Alonso F, Riente P, Yus M (2008) Eur J Org Chem 4908. https://doi.org/10.1002/ejoc.200800729
Cui X, Zhang Y, Shi F, Deng Y (2011) Chem Eur J 17:1021
Tan DW, Li HX, Zhu DL, Li HY, Young DJ, Yao JL, Lang JP (2018) Org Lett 20:608
Lawal NS, Bala MD (2020) J Mol Struct 1200:127124
Kadwa E, Friedrich HB, Bala MD (2017) Inorg Chim Acta 463:112
Mathew P, Neels A, Albrecht M (2008) J Am Chem Soc 130:13534
Mncube SG, Bala MD (2016) J Mol Liq 215:396
Dhimba G, Lawal NS, Bala MD (2019) J Mol Struct 1179:100
Abubakar S, Ibrahim H, Bala MD (2019) Inorg Chim Acta 484:276
Lake BRM, Willans CE (2014) Organometallics 33:2027
Mncube SG, Bala MD (2019) Trans Met Chem 44:145
Ihaumeer-Laulloo BS, Bhowon MG (2003) Indian J Chem 42A:2536
Bagihalli GB, Patil SA, Badami PS (2009) J Iran Chem Soc 6:259
Singh K, Singh DP, Barwa MS, Tyagi P, Mirza Y (2006) J Enzyme Inhib Med Chem 21:749
Singh K, Kumar Y, Puri P, Sharma C, Aneja KR (2017) Arab J Chem 10:S978
Grusenmeyer TA, King AW, Mague JT, Rack JJ, Schmehl RH (2014) Dalton Trans 43:17754
Hauwert P, Boerleider R, Warsink S, Weigand JJ, Elsevier CJ (2010) J Am Chem Soc 132:16900
Ibrahim H, Bala MD (2016) New J Chem 40:6986
Chen J, Yuan T, Hao W, Cai M (2011) Tetrahedron Lett 52:3710
Dudev T, Lim C (1998) J Am Chem Soc 120:4450
Aihara J (1992) Sci Am 266:62
Lal S, Diez-Gonzalez S (2011) J Org Chem 76:2367
Cozzi PG (2004) Chem Soc Rev 33:410
Acknowledgements
We acknowledge financial support from the University of KwaZulu-Natal, ESKOM (TESP 2019) and the NRF. NSL thanks Ahmadu Bello University for a paid study fellowship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lawal, N.S., Ibrahim, H. & Bala, M.D. Cu(I) mediated hydrogen borrowing strategy for the α-alkylation of aryl ketones with aryl alcohols. Monatsh Chem 152, 275–285 (2021). https://doi.org/10.1007/s00706-021-02735-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-021-02735-5