Abstract
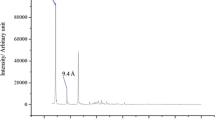
This work reports for the first time the use of titanate nanotubes (TiNT) as adsorbent structure for Hg2+ ions and its use in construction of mercury-based electrodes for analytical applications. TiNTs with diameter of around 15 nm and length between 40 and 200 nm were obtained by hydrothermal alkaline synthesis and characterized by FTIR, DRX, and TEM. A carbon paste electrode modified with 15% (w/w) of TiNT was used for spontaneous mercury ions incorporation, by simply immerging in a Hg2+ solution for 120 s. “Mercury isles” were obtained by electrochemical reduction of adsorbed Hg2+ ions. Under the best optimized conditions, the proposed device was evaluated for the determination of Zn2+ ions in pharmaceutical samples. A linear relationship of anodic peak current and Zn2+ ions concentration was observed at a range of 4.0–20 µmol dm−3, with sensitivity of 0.54 μA dm3 μmol−1, limit of detection and limit of quantification of 1.2 µmol dm−3 and 4.0 µmol dm−3, respectively. Satisfactory agreement with a comparative method showed the useful application of the sensor, with the advantage of the Zn preconcentration step at open circuit potential condition. This strategy allows the use of several electrodes at the same time, which characterize this device as a feasible passive sampler.
Graphic abstract





Similar content being viewed by others
References
Bavykin DV, Carravetta M, Kulak AN, Walsh FC (2010) Chem Mater 22:2458
Abdullah M, Kamarudin SK (2017) Renew Sustain Energy Rev 76:212
Han CH, Hong DW, Kim IJ, Gwak J, Han SD, Singhb KC (2007) Sens Actuators B Chem 128:320
Mahlambi MM, Ngila CJ, Mamba BB (2015) J Nanomater 2015:790173
Zhao R, Liu X, Zhang J, Zhu J, Wong DKY (2015) Electrochim Acta 163:64
Yang M, Wang J, Li H, Zheng JG, Wu NN (2008) Nanotechnology 19:075502
Sheng G, Yang S, Sheng J, Zhao D, Wang X (2011) Chem Eng J 168:178
Souza JS, Carvalho-Jr WM, Souza FL, Ponce-de-Leon C, Bavykin DV, Alves WA (2016) J Mater Chem A 4:944
Etienne M, Bessiere J, Walcarius A (2001) Sens Actuators B Chem 76:531
Oliveira PR, Lamy-Mendes AC, Rezende EIP, Mangrich AS, Marcolino-Junior LH, Bergamini MF (2015) Food Chem 171:426
Al-Harbi EA, El-Shahawi MS (2018) Electroanalysis 30:1837
Biçer E, Arat C (2008) J Chil Chem Soc 53:1734
Heyrovský J, Kůta J (2013) Principles of polarography. Elsevier, Amsterdam
Gajdar J, Horakova E, Barek J, Fischer J, Vyskočil V (2016) Electroanalysis 28:2659
Vyskočil V, Barek J (2009) Crit Rev Anal Chem 39:173
Kudr J, Nguyen HV, Gumulec J, Nejdl L, Blazkova I, Ruttkay-Nedecky B, Hynek D, Kynicky J, Adam V, Kizek R (2014) Sensors (Switzerland) 15:592
Temerk Y, Ibrahim M, Ibrahim H, Kotb M (2016) J Electroanal Chem 760:135
Hambidge KM, Miller LV, Westcott JE, Krebs NF (2008) J Nutr 138:2363
Jen M, Yan AC (2010) Clin Dermatol 28:669
Chaiyo S, Mehmeti E, Žagar K, Siangproh W, Chailapakul O, Kalcher K (2016) Anal Chim Acta 918:26
Lu Z, Zhang J, Dai W, Lin X, Ye J, Ye J (2017) Microchim Acta 184:4731
JCPDS-International Center for Diffraction Data, PDF 21-1272
JCPDS-International Center for Diffraction Data, PDF 00-047-0124
Viana BC, Ferreira OP, Souza Filho AG, Hidalgo AA, Mendes-Filho J, Alves OL (2009) J Braz Chem Soc 20:167
Booij K, Vrana B, Huckins JN (2007) Compr Anal Chem 48:141
de Oliveira PR, Lamy-Mendes AC, Gogola JL, Mangrich AS, Marcolino-Junior LH, Bergamini MF (2015) Electrochim Acta 151:525
Shahbazi Y, Ahmadi F, Fakhari F (2016) Food Chem 192:1060
Švancara I, Pravda M, Hvizdalová M, Vytřas K, Kalcher K (1994) Electroanalysis 6:663
Da Silva CL, Masini JC (2000) Fresenius J Anal Chem 367:284
Kasuga T, Hiramatsu M, Hoson A, Sekino T, Niihara K (1998) Langmuir 14:3160
Acknowledgments
The authors are grateful to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação Araucária and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bindewald, E.H., Angelo, E., Kleinert, E. et al. Mercury isles in titanate nanotubes: a new strategy for using mercury electrodes in analytical application. Monatsh Chem 151, 1485–1491 (2020). https://doi.org/10.1007/s00706-020-02691-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-020-02691-6