Abstract
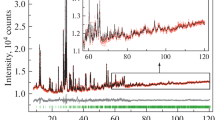
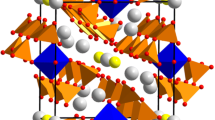
Single crystals of the new compound, NaMn2V3O10, were grown by conventional solid-state reaction. The crystal structure of the synthesized sample was determined using single-crystal X-ray diffraction data. NaMn2V3O10 crystallizes in the triclinic system with space group P\(\stackrel{-}{1}\) and cell parameters a = 6.854 (2) Å, b = 6.877 (2) Å, c = 9.716 (2) Å, α = 100.604 (1)°, β = 104.169 (1)°, and γ = 101.390 (1)°. The structure of this vanadate built up from the connection of V3O10 groups and MnO6 octahedra via corners leading to an open three-dimensional framework delimiting tunnels along [001] direction, where the Na+ cations are localized. The powder of NaMn2V3O10 was prepared by Pechini method. The magnetic measurement has been evidenced by Curie–Weiss behavior above 4.5 K with θ = − 25.46 K demonstrating the occurrence of predominant antiferromagnetic interactions between Mn2+ cations.
Graphic abstract











Similar content being viewed by others
References
Thackeray MM, Johnson CS, Vaughey JT, Li N, Hackney SA (2005) J Mater Chem 15:2257
Liu JCW, Oh P, Liu X, Lee MJ, Cho W, Chae S, Kim Y (2015) Angew Chem Int Ed 54:4440
Zhang D, Xi S, Li G, Li B, Fan J, Liu X, Chen D, Li L (2019) J Power Sources 426:197
Hua K, Li X, Fang D, Yi J, Bao R, Luo Z (2018) Appl Surf Sci 447:610
Hua K, Li X, Fu Z, Fang D, Bao R, Yi J, Luo Z (2019) J Solid State Chem 273:287
Ni M, Leung MKH, Leung DYC, Sumathy K (2007) Renew Sustain Energy Rev 11:401
Pan JH, Dou H, Xiong Z, Xu C, Ma J, Zhao XS (2010) J Mater Chem 20:4512
Ouyang S, Li Z, Ouyang Z, Yu T, Ye J, Zou Z (2008) J Phys Chem 6:3134
Teja AS, Koh P (2009) Prog Cryst Growth Charact Mater 55:22
Woo K, Hong J, Choi S, Lee HW, Ahn JP, Kim CS, Lee SW (2004) Chem Mater 8:2814
Pei LZ, Pei YQ, Xie YK, Fan CG, Yu HY (2013) CrystEngComm 15:1729
Ben Yahia H, Gaudin E, Boulahya K, Darriet J, Son WJ, Whangbo MH (2010) Inorg Chem 49:8578
Hua K, Li X, Fang D, Yi J, Wu X, Luo Z (2018) Mater Sci Eng B 238–239:26
Tan L, Liu H (2010) Inorg Mater 46:201
Brown ID, Altermatt D (1985) Acta Cryst B41:244
Ayed B, Haddad A (2008) Acta Cryst E64:i21
Ben Smida Y, Marzouki R, Guesmi A, Georges S, Zid MF (2015) J Solid State Chem 221:132
Wang X, Liu Z, Ambrosini A, Maignan A, Stern LC, Poeppelmeier RK, Dravid PV (2000) Solid State Sci 2:99
Sanjeewa LD, McGuire MA, Pellizzeri TMS, McMillen CD, Ovidiu Garlea V, Willett D, Chumanov G, Kolis JW (2016) J Solid State Chem 241:30
Dwibedi D, Araujo BR, Chakraborty S, Shanbogh PP, Sundaram NG, Ahuja R, Barpanda P (2015) J Mater Chem A 3:18564
Hadouchi M, Assani A, Saadi M, Saadoune I, Lahmar A, Bouyanfif H, El Marssi M, El Ammari L (2018) J Inorg Organomet Polym 28:2854
Benhsina E, Assani A, Saadi M, El Ammari L (2016) Acta Cryst E72:220
Bruker (2009) APEX2, SAINT, and SADABS. Bruker AXS Inc Madison, Wisconsin
Sheldrick GM (2015) Acta Cryst C71:3
Sheldrick GM (2015) Acta Cryst A71:3
Farrugia LJ (1999) J Appl Cryst 32:849
Brandenburg K (2006) DIAMOND. Crystal Impact GbR, Bonn, Germany
Petříček V, Dušek M, Palatinus L (2014) Z Kristallogr 229:345–352
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Benhsina, E., Assani, A., Saadi, M. et al. A new sodium- and manganese-based trivanadate NaMn2V3O10: synthesis, structural and magnetic insights. Monatsh Chem 151, 677–684 (2020). https://doi.org/10.1007/s00706-020-02608-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-020-02608-3