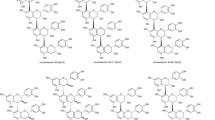
Abstract
This study aimed to achieve the synthesis of dimers containing cholesterol and ergosterol using click reaction and to perform biologic evaluations. For this purpose, cholesterol and ergosterol were converted to their esters. Three ergosterol esters were reported as novel compounds. To be used as linkers, furan and thiophene derivatives containing azide groups were synthesized. Of these, 2,5-bis(azidomethyl)thiophene was reported to be a novel compound. The obtained compounds were converted to dimers using a click reaction and 12 novel dimers were obtained as a result. The synthesized compounds were examined to evaluate their biologic activity against MCF-7 and HT29 cancer cells and MEF healthy cells. As a result of the biologic evaluation, the most effective compound against the MCF-7 cancer cell line was [2,5-furandiylbis(methylene)]bis(cholest-5-en-3-yl1H-1,2,3-triazole-4-pentanoate) with 205.06 µM concentration and [2,5-furandiylbis(methylene)]bis(ergosta-5,7,22-trien-3-yl1H-1,2,3-triazole-4-propanoate) with 159.5 µM concentration was the most effective for the HT29 cancer cell line.
Graphic abstract


Similar content being viewed by others
References
Zanatta N, Alves SH, Coelho HS, Borchhardt DM, Machado P, Flores KM, Da Silva FM, Spader TB, Santurio JM, Bonacorso HG, Martins MAP (2007) Bioorg Med Chem 15:1947
Gramec D, Mašič LP, Dolenc MS (2014) Chem Res Toxicol 27:1344
Liu KKC, Zhu J, Smith GL, Yin MJ, Bailey S, Chen JH, Hu Q, Huang Q, Li C, Li QJ, Marx MA, Paderes G, Richardson PF, Sach NW, Walls M, Wells PA, Zou A (2011) ACS Med Chem Lett 2:809
Malladi S, Nadh RV, Babu KS, Babu PS (2017) Beni-Suef Univ J Basic Appl Sci 6:345
Masteri-Farahani M, Modarres M (2017) Colloids Surf A 529:886
Wu C, Wang CZ, Zhu Q, Zeng X, Redshaw C, Yamato T (2018) Sens Actuators B 254:52
Artyushin OI, Sharova EV, Vinogradova NM, Genkina GK, Moiseeva AA, Klemenkova ZS, Orshanskaya IR, Shtro AA, Kadyrova RA, Zarubaev VV, Yarovaya OI, Salakhutdinov NF, Brel VK (2017) Bioorg Med Chem Lett 27:2181
Garudachari B, Isloor AM, Satyanarayana MN, Fun HK, Hegde G (2014) Eur J Med Chem 74:324
Kaushik CP, Kumar K, Lal K, Narasimhan B, Kumar A (2016) Monatsh Chem 147:817
Zhang Y, Lv Z, Zhong H, Geng D, Zhang M, Zhang T, Li Y, Li K (2012) Eur J Med Chem 53:356
Ameen MA, Ahmed EK, Ramadan M, El-Naby HAA, Abdel-Haseeb AA (2017) Monatsh Chem 148:1513
Karabulut HRF, Karatavuk AO, Ozyildirim H, Doğanlar O, Doğanlar ZB (2020) Med Chem Res 29:643
Zhang YL, Li YF, Shi XL, Yu B, Shi YK, Liu HM (2016) Steroids 115:147
Sribalan R, Padmini V, Lavanya A, Ponnuvel K (2016) Saudi Pharm J 24:658
Aly MRES, Saad HA, Mohamed MAM (2015) Bioorg Med Chem Lett 25:2824
Mydock-McGrane L, Rath NP, Covey DF (2014) J Org Chem 79:5636
Lin M, Li H, Zhao Y, Cai E, Zhu H, Gao Y, Liu S, Yang H, Zhang L, Tang G (2017) Steroids 122:9
Okamoto K, Watanabe M, Sakata N, Murai M, Ohe K (2013) Org Lett 15:5810
Boeck F, Kribber T, Xiao L, Hintermann L (2011) J Am Chem Soc 133:8138
Xi Y, Dong B, McClain EJ, Wang Q, Gregg TL, Akhmedov NG, Petersen JL, Shi X (2014) Angew Chem Int Ed 53:4657
Cottier L, Descotes G, Soro Y (2003) Synth Commun 33:4285
Dow M, Marchetti F, Abrahams KA, Vaz L, Besra GS, Warriner S, Nelson A (2017) Chem Eur J 23:7207
Acknowledgements
We thank Trakya University for its support through project number TUBAP 2017/113. We are also grateful to TUBİTAK Department for Support of Scientists (BİDEB) for its support in the framework of a 2211-A national doctoral bursary during this doctoral study. We thank Trakya University Technology Research Development Application and Research Center (TUTAGEM) for performing biologic activity tests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Karatavuk, A.O., Karabulut, H.R.F. Synthesis of novel dimers containing cholesterol and ergosterol using click reaction and their anti-proliferative effects. Monatsh Chem 151, 837–844 (2020). https://doi.org/10.1007/s00706-020-02594-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-020-02594-6