Abstract
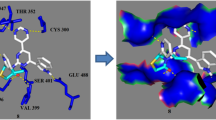
New azidosulfonamide–chalcone derivatives were designed and synthesized. Their structures were elucidated by 1H and 13C NMR spectral analyses, in addition to elemental analyses. The synthesized derivatives were tested for their antimicrobial activity against a wide variety of Gram-positive, Gram-negative, and fungal strains. Three azidosulfonamide–chalcones showed relatively broad activity against tested strains. Two compounds exhibited eminent antibacterial activity toward S. aureus, M. luteus, and S. marcens (better than ampicillin trihydrate). The synthesized compounds exhibited moderate activity against K. pneumonia and a lower ability to inhibit E. coli growth. Among six tested fungal species, the most potent derivatives demonstrated strong activity toward only two of the fungal strains (T. rubrum and G. candidum). Assessment of drug-likeness, bioavailability, and promiscuity indicated that the compounds are viable drug candidates. In silico molecular docking analysis revealed that the synthesized azidosulfonamide–chalcones successfully occupied pterin-binding site of the dihydropteroate synthase (DHPS), implying that the prepared compounds could exert their activity by the inhibition of the microbial DHPS enzyme. These results provided essential information for the prospective design of more effective antimicrobial compounds.
Graphic abstract





Similar content being viewed by others
References
Ahmed N, Konduru NK, Owais M (2019) Arab J Chem 12:1879
Payne DJ, Gwynn MN, Holmes DJ, Pompliano DL (2007) Nat Rev Drug Discov 6:29
Sivakumar PM, Ganesan S, Veluchamy P, Doble M (2010) Chem Biol Drug Des 76:407
Phetsang W, Blaskovich MA, Butler MS, Huang JX, Zuegg J, Mamidyala SK, Ramu S, Kavanagh AM, Cooper MA (2014) Bioorg Med Chem 22:4490
Butler MS, Blaskovich MA, Cooper MA (2017) J Antibiot 70:3
Zha G-F, Wang S-M, Rakesh K, Bukhari S, Manukumar H, Vivek H, Mallesha N, Qin H-L (2019) Eur J Med Chem 162:364
El-Kardocy A, Mustafa M, Ahmed ER, Mohamady S, Mostafa YA (2019) Med Chem Res 28:2088
Ghorab MM, Al-Said MS (2012) Arch Pharmacal Res 35:987
El-Din MMG, El-Gamal MI, Abdel-Maksoud MS, Yoo KH, Oh C-H (2015) Eur J Med Chem 90:45
Ghorab MM, Ragab FA, Heiba HI, Agha HM, Nissan YM (2012) Arch Pharmacal Res 35:59
Ghorab MM, Ismail ZH, Abdalla M, Radwan AA (2013) Arch Pharmacal Res 36:660
Arshad M (2018) Int J Pharm Sci Res 9:35
Lavanya R (2017) Int J Pharm Sci Invent 6:1
Askar F, Aldhalf Y, Jinzeel N (2017) Int J Chem Sci 15:173
Fathalla OA, Zaghary WA, Radwan HH, Awad SM, Mohamed MS (2002) Arch Pharmacal Res 25:258
Bahekar SP, Hande SV, Agrawal NR, Chandak HS, Bhoj PS, Goswami K, Reddy M (2016) Eur J Med Chem 124:262
Basanagouda M, Shivashankar K, Kulkarni MV, Rasal VP, Patel H, Mutha SS, Mohite AA (2010) Eur J Med Chem 45:1151
Uddin MJ, Rao PP, Knaus EE (2003) Bioorg Med Chem 11:5273
Pinney KG, Mejia MP, Villalobos VM, Rosenquist BE, Pettit GR, Verdier-Pinard P, Hamel E (2000) Bioorg Med Chem 8:2417
Agard NJ, Baskin JM, Prescher JA, Lo A, Bertozzi CR (2006) ACS Chem Biol 1:644
Meyer D, Smeilus T, Pliatsika D, Mousavizadeh F, Giannis A (2017) Bioorg Med Chem 25:6098
Van Ostrand R, Jacobsen C, Delahunty A, Stringer C, Noorbehesht R, Ahmed H, Awad AM (2017) Nucleosides. Nucleotides Nucleic Acids 36:181
Nasr T, Bondock S, Eid S (2016) J Enzyme Inhib Med Chem 31:236
Yun M-K, Wu Y, Li Z, Zhao Y, Waddell MB, Ferreira AM, Lee RE, Bashford D, White SW (2012) Science 335:1110
Lipinski CA (2000) J Pharmacol Toxicol Methods 44:235
Lipinski CA, Lombardo F, Dominy BW, Feeney PJ (1997) Adv Drug Deliv Rev 23:3
Daina A, Michielin O, Zoete V (2017) Sci Rep 7:42717
Martin YC (2005) J Med Chem 48:3164
Yang JJ, Ursu O, Lipinski CA, Sklar LA, Oprea TI, Bologa CG (2016) J Cheminform 8:29
Mustafa M, Anwar S, Elgamal F, Ahmed ER, Aly OM (2019) Eur J Med Chem 183:111697
Clinical and Laboratory Standards Institute (2015) Performance standards for antimicrobial susceptibility testing. In: 25th informational supplement, M100-S25, vol 35, no 3. CLSI, Payne
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mustafa, M., Mostafa, Y.A. A facile synthesis, drug-likeness, and in silico molecular docking of certain new azidosulfonamide–chalcones and their in vitro antimicrobial activity. Monatsh Chem 151, 417–427 (2020). https://doi.org/10.1007/s00706-020-02568-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-020-02568-8