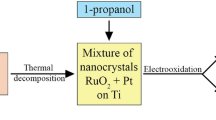
Abstract
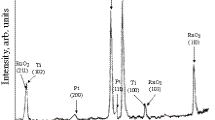
Thermally prepared catalytic coatings on a titanium substrate were composed of a mixture of nanocrystals of metallic Pt and RuO2 of rutile structure and used for electrooxidation of formaldehyde. The size of the RuO2 nanocrystals increased, whereas those of Pt decreased with increasing the content of RuO2 in the mixture. At more positive potentials, the maximum catalytic activities showed the coatings with lower content of RuO2. Mechanism of formaldehyde oxidation was derived to show two reaction pathways. In the first one, H2C(OH)2 was directly oxidized to CO2, whereas COad was formed in the latter. COad is strongly adsorbed on Pt atoms, which causes blocking of these atoms and thus, preventing direct dehydrogenation of H2C(OH)2 to CO2. The overall catalytic effect of the mixture of nanocrystals was caused by the bifunctional mechanism. Thus, the Ru atoms formed the oxy species at more negative potentials than Pt. These oxy species oxidized the COad intermediates, bound to adjacent Pt atoms and accordingly, discharged them for dehydrogenation of new molecules of H2C(OH)2.
Graphic abstract











Similar content being viewed by others
References
Yu X, Pickup PG (2008) J Power Sources 182:124
Rice C, Ha S, Masel RI, Waszczuk P, Wieckowski A, Barnard T (2002) J Power Sources 111:83
Korzeniewski C, Childers CL (1998) J Phys Chem B 102:489
Guthrie JP (1975) Can J Chem 53:898
Spasojević MD, Adžić RR, Despić AR (1980) J Electroanal Chem 109:261
Adžić RR, Hofman MI, Dražić DM (1980) J Electroanal Chem 110:361
Kazarinov VE, Vassiliev YB, Andreev VN, Kuliev SA (1981) J Electroanal Chem 123:345
Olivi P, Bulhões LOS, Léger J-M, Hahn F, Beden B, Lamy C (1994) J Electroanal Chem 370:241
Olivi P, Bulhões LOS, Léger J-M, Hahn F, Beden B, Lamy C (1996) Electrochim Acta 41:927
Koper MTM, Hachkar M, Beden B (1996) J Chem Soc Faraday Trans 92:3975
Miki A, Ye S, Senzaki T, Osawa M (2004) J Electroanal Chem 563:23
Samjeské G, Miki A, Osawa M (2007) J Phys Chem C 111:15074
Avramov-Ivic M, Adzic RR, Bewick A, Razaq M (1988) J Electroanal Chem, Interfacial. Electrochem 240:161
Kitamura F, Takahashi M, Ito M (1986) Chem Phys Lett 123:273
Nishimura K, Ohnishi R, Kunimatsu K, Enyo M (1989) J Electroanal Chem, Interfacial. Electrochem 258:219
Sun SG, Lu GQ, Tian ZW (1995) J Electroanal Chem 393:97
Bełtowska-Brzezinska M, Heitbaum J, Vielstich W (1985) Electrochim Acta 30:1465
Xu Y, Schell M (1990) J Phys Chem 94:7137
Nakabayashi S, Kira A (1992) J Phys Chem 96:1021
Batista EA, Iwasita T (2006) Langmuir 22:7912
Osawa M (2001) Surface-Enhanced Infrared Absorption. In: Kawata S (ed), Near-Field Optics and Surface Plasmon Polaritons. Top. Appl. Phys, vol 81. Springer, Berlin, p 163.
Zhang Y, Zhang M, Cai Z, Chen M, Cheng F (2012) Electrochim Acta 68:172
Guo Y, Xu YT, Gao GH, Wang T, Zhao B, Fu XZ, Sun R, Wong CP (2015) Catal Commun 58:40
Yan RW, Jin BK (2013) Chin Chem Lett 24:159
Nellaiappan S, Kumar AS, Nisha S, Pillai KC (2017) Electrochim Acta 249:227
Bansal V, Li V, O’Mullane AP, Bhargava SK (2010) CrystEngComm 12:4280
Yu Y, Jia M, Tian H, Hu J (2014) J Power Sources 267:123
Li Z, Lu X, Li B, Bai L, Wang Q (2015) ECS Electrochem Lett 4:H24
Trivedi D, Crosse J, Tanti J, Cass AJ, Toghill KE (2018) Sens Actuators B 270:298
Hassaninejad-Darzi SK (2014) J Electroceram 33:252
Hasanzadeh M, Khalilzadeh B, Shadjou N, Karim-Nezhad G, Saghatforoush L, Kazeman I, Abnosi MH (2010) Electroanalysis 22:168
Yang L, Zhao F, Xiao F, Zeng B (2011) Anal Bioanal Electrochem 3:175
Raoof JB, Hosseini SR, Ojani R, Aghajani S (2015) J Mol Liq 204:106
Safavi A, Momeni S, Tohidi M (2012) Electroanalysis 24:1981
Safavi A, Farjami F (2011) Electroanalysis 23:1842
Miao F, Tao B (2013) J Nanosci Nanotechnol 13:3104
de Lima RB, Massafera MP, Batista EA, Iwasita T (2007) J Electroanal Chem 603:142
Touny AH, Tammam RH, Saleh MM (2018) Appl Catal B: Environ 224:1017
Momeni S, Sedaghati F (2018) Microchem J 143:64
Spasojevic M, Ribic-Zelenovic L, Spasojevic M, Trisovic T (2019) Russ J Electrochem 55:1350
Burke LD, O'Neill JF (1979) J Electroanal Chem, Interfacial. Electrochem 101:341
Franaszczuk K, Sobkowski J (1992) J Electroanal Chem 327:235
Spasojević MD, Krstajić NV, Jakšić MM (1987) J Mol Catal 40:311
Spasojevic M, Ribic-Zelenovic L, Spasojevic P (2012) Ceram Int 38:5827
Spasojevic M, Krstajic N, Spasojevic P, Ribic-Zelenovic L (2015) Chem Eng Res Des 93:591
Hadzi-Jordanov S, Angerstein-Kozlowska H, Vukovic M, Conway BE (1977) J Phys Chem 81:2271
Ticanelli E, Beery JG, Paffett MT, Gottesfeld S (1989) J Electroanal Chem, Interfacial. Electrochem 258:61
Capon A, Parson R (1973) J Electroanal Chem, Interfacial. Electrochem. 44:1
Wakisaka M, Mitsui S, Hirose Y, Kawashima K, Uchida H, Watanabe M (2006) J Phys Chem B 110:23489
Rigsby MA, Zhou WP, Lewera A, Duong HT, Bagus PS, Jaegermann W, Hunger R, Wieckowski A (2008) J Phys Chem C 112:15595
Garrick TR, Diao W, Tengco JM, Stach EA, Senanayake SD, Chen DA, Monnier JR, Weidner JW (2016) Electrochim Acta 195:106
Tian M, Shi S, Shen Y, Yin H (2019) Electrochim Acta 293:390
Acknowledgements
This work has been supported by the Ministry of Education and Science of the Republic of Serbia through project Ref. No. 172057
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Spasojevic, M., Spasojevic, M. & Ribic-Zelenovic, L. A catalyst coated electrode for electrochemical formaldehyde oxidation. Monatsh Chem 151, 33–43 (2020). https://doi.org/10.1007/s00706-019-02533-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-019-02533-0