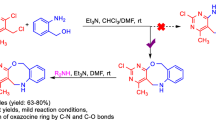
Abstract
An efficient and facile method for the synthesis of novel naphthooxazocines from 2-hydroxy-1,4-naphthoquinone and quinolinium salts at room temperature is described. The reaction proceeds through C-alkylation and intramolecular O-alkylation, affording the desired products in good yields.
Graphical abstract


Similar content being viewed by others
References
Shigemori H, Kagata T, Ishiyama H, Morah F, Ohsaki A, Kobayashi J (2003) Chem Pharm Bull 51:58
Zhang CR, Liu HB, Dong SH, Zhu JY, Wu Y, Yue JM (2009) Org Lett 11:4692
Lounasmaa M, Karvinen E (1993) Heterocycles 36:751
Klohs MW, Draper MS, Petracek FJ, Ginzel KH, Re ON (1972) Arzneim-Forsch (Drug Res) 22:132
Mishra JK, Samanta K, Jain M, Dikshit M, Panda G (2010) Biorgan Med Chem Lett 20:244
Seto S, Tanioka A, Ikeda M, Izawa S (2005) Biorgan Med Chem Lett 15:1485
Sahn JJ, Martin SF (2011) Tetrahedron Lett 52:6855
Narjes F, Crescenzi B, Ferrara M, Habermann J, Colarusso S, del Rosario MRF, Stansfield I, Mackay AC, Conte I, Ercolani C, Zaramella S, Palumbi MC, Meuleman P, Leroux-Roels G, Giuliano C, Fiore F, Di Marco S, Baiocco P, Koch U, Migliaccio G, Altamura S, Laufer R, De Francesco R, Rowley M (2011) J Med Chem 54:289
Broggini G, Bruch L, Garanti L, Zecchi G (1994) J Chem Soc Perkin Trans 1:433
Gurubrahamam R, Nagarajua K, Chen K (2018) Chem Commun 54:6048
Sivaguru P, Parameswaran K, Lalitha A (2016) Tetrahedron Lett 57:2549
Zhang E, Zhang X, Wei W, Wang D, Cai Y, Xu T, Yana M, Yong Z (2015) RSC Adv 5:5288
Van Otterlo WAL, Morgans GL, Khanye SD, Aderibigbe BAA, Michael JP, Billing DG (2004) Tetrahedron Lett 45:9171
Klapars A, Parris S, Anderson KW, Buchwald SL (2004) J Am Chem Soc 126:3529
Mishra JK, Panda G (2007) J Comb Chem 9:321
Yang T, Lin C, Fu H, Jiang Y, Zhao Y (2005) Org Lett 7:4781
Tietze LF, Gordon B, Kersten GM (2006) Domino reactions in organic synthesis. Wiley, Weinheim
Hayashi Y (2016) Chem Sci 7:866
Guo HC, Ma JA (2006) Angew Chem Int Ed 45:354
Hussian MM, Walsh PJ (2008) Acc Chem Res 41:883
Moghaddam FM, Mirjafary Z, Saeidian H, Taheri S, Doulabi M, Kiamehr M (2010) Tetrahedron 66:134
Moghaddam FM, Taheri S, Mirjafary Z, Saeidian H (2010) Synlett 1:123
Moghaddam FM, Saeidian H, Kiamehr M, Mirjafary Z, Taheri S (2010) Arkivoc 11:91
Schmidt A, Michalik D, Rotzoll S, Ullah E, Fischer C, Reinke H, Görls H, Langer P (2008) Org Biomol Chem 6:2804
Bazin M, Kuhn C (2005) J Comb Chem 7:302
Nájera C, Sansano JM, Yus M (2015) Org Biomol Chem 13:8596
Liu Y, Zhang Y, Shen YM, Hu HW, Xu JH (2010) Org Biomol Chem 8:2449
Wu L, Sun J, Yan CG (2012) Org Biomol Chem 10:9452
Zhao J, Li P, Wu C, Chen H, Ai W, Sun R, Ren H, Larock RC, Shi F (2012) Org Biomol Chem 10:1922
Noushini S, Mahdavi M, Firoozpour L, Moghimi S, Shafiee A, Foroumadi A (2015) Tetrahedron 71:6272
Firoozpour L, Nikookar H, Moghimi S, Mahdavi M, Asadipour A, Ranjbar PR, Foroumadi A (2017) Heterocycl Commun 23:305
Sadat-Ebrahimi SE, Katebi S, Pirali-Hamedani M, Moghimi S, Yahya-Meymandi A, Mahdavi M, Shafiee A, Foroumadi A (2016) Heterocycl Commun 22:247
Yahyavi H, Heravi MM, Mahdavi M, Foroumadi A (2018) Tetrahedron Lett 59:94
Yahya-Meymandi A, Nikookar H, Moghimi S, Mahdavi M, Firoozpour L, Asadipour A, Ranjbar PR, Foroumadi A (2017) J Iran Chem Soc 14:771
Almasirad A, Firoozpour L, Nejati M, Edraki N, Firuzi O, Khoshneviszadeh M, Mahdavi M, Moghimi S, Safavi M, Shafiee A, Foroumadi A (2016) Z Naturforsch B 71:205
Rezaei Z, Moghimi S, Javaheri R, Asadi M, Mahdavi M, Shabani S, Edraki N, Firuzi O, Safavi M, Amini M, Asadipour A, Zeinalzadeh E, Firoozpour L, Foroumadi A (2017) Lett Drug Des Discov 14:1138
Loska R, Majcher M, Makosza M (2007) J Org Chem 72:5574
Acknowledgements
This work was supported and funded by a grant from the research council of Tehran University of Medical Sciences (TUMS).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Madani Qamsari, F., Moradi, S., Foroumadi, A. et al. Tandem synthesis of benzo[d]naphtho[2,3-g][1,3]oxazocine-8,13(6H,14H)-dione derivatives. Monatsh Chem 150, 347–352 (2019). https://doi.org/10.1007/s00706-018-2322-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-018-2322-8