Abstract
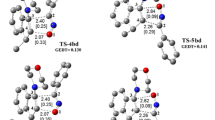
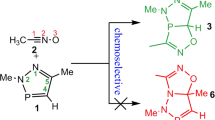
The double 1,3-dipolar cycloaddition reaction between nitrilimine and allenoate, experimentally investigated by Guo and co-workers, was theoretically studied at the B3LYP/6-311G** and wB97XD/6-311G** computational levels in both gas phase and dichloromethane solution. The results indicated that the formation of the experimentally reported product is clearly explained by the analysis of the calculated Fukui function reactivity indices as well as transition states studies. Frontier molecular orbitals analysis showed that the HOMO orbital of nitrilimine as donor is also the frontier effective-for-reaction molecular orbital (FERMO). Finally, on the basis of the Wiberg bond indexes and AIM analysis, it was found that all of the reactive channels proceed through an asynchronous concerted mechanism.
Graphical abstract








Similar content being viewed by others
References
Huisgen R (1963) Angew Chem Int Ed 2:565
Hashimoto T, Maruoka K (2015) Chem Rev 115:5366
Bdiri B, Zhao B-J, Zhou Zh-M (2017) Tetrahedron Asymmetry 28:876
Moss GP (1999) Pure Appl Chem 71:531
Yavari I, Nematpour M, Sodagar E (2015) Monatsh Chem 146:2135
Abdou WM, Ganoub NA, Sabry E (2016) Monatsh Chem 147:619
Dawood KM (2005) J Heterocycl Chem 42:221
Monteiro Â, Gonçalves LM, Santos MMM (2014) Eur J Med Chem 79:266
Farghaly TA, Gomha SM, Dawood KM, Shaaban MR (2016) RSC Adv 6:17955
Dadiboyena S, Valente EJ, Hamme AT II (2014) Tetrahedron Lett 55:2208
Santos MMM (2014) Tetrahedron 70:9735
Liu H, Jia H, Wang B, Xiao Y, Guo H (2017) Org Lett 19:4714
Soleymani M, Dashti Khavidaki H (2017) Comp Theor Chem 1112:37
Memarian HR, Soleymani M, Sabzyan H (2012) J Iran Chem Soc 9:805
Memarian HR, Soleymani M, Sabzyan H, Bagherzadeh M, Ahmadi H (2011) J Phys Chem A 115:8264
Memarian HR, Sabzyan H, Soleymani M, Habibi MH, Suzuki T (2011) J Mol Struct 998:91
Geerlings P, De Proft F, Langenaeker W (2003) Chem Rev 103:1793
Yang W, Mortier WJ (1986) J Am Chem Soc 108:5708
Domingo LR, Pérez P, Sáez JA (2013) RSC Adv 3:1486
Chamorro E, Pérez P, Domingo LR (2013) Chem Phys Lett 582:141
Domingo LR (2014) RSC Adv 4:32415
Da Silva RR, Ramalho TC, Santos JM, Figueroa-Villar JD (2006) J Phys Chem A 110:1031
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchia, HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels A, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, Revision E.01. Gaussian Inc, Wallingford, CT
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785
Chai JD, Head-Gordon M (2008) Phys Chem Chem Phys 10:6615
Barone V, Cossi M (1998) J Phys Chem A 102:1995
Schlegel HB (1982) J Comput Chem 3:214
Peng C, Ayala PY, Schlegel HB, Frisch MJ (1996) J Comput Chem 17:49
Gonzalez C, Schlegel HB (1989) J Chem Phys 90:2154
Gonzalez C, Schlegel HB (1990) J Phys Chem 94:5523
Eyring H (1935) J Chem Phys 3:107
Reed AE, Curtiss LA, Weinhold F (1988) Chem Rev 88:899
Carpenter JE, Weinhold F (1988) J Mol Struct 169:41
Domingo LR, Perez P, Ortega DE (2013) J Org Chem 78:2462
Parr RG, Szentpaly LV, Liu S (1999) J Am Chem Soc 121:1922
Acknowledgements
I am thankful to the Research Council and Office of Graduate Studies of the University of Ayatollah Alozma Borujerdi for their financial support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Soleymani, M. DFT study of double 1,3-dipolar cycloaddition of nitrilimines with allenoates. Monatsh Chem 149, 2183–2193 (2018). https://doi.org/10.1007/s00706-018-2311-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-018-2311-y