Abstract
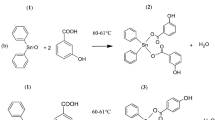
As a part of our quest to develop new bioactive bisphosphonic acids, we synthesized a series of bis(α-aminobisphosphonic acids) in good yields (66–78%) as new entities for treating malignant melanoma. The reaction of the Schiff bases, 1,4-pheneylenediimines with the Horner–Emmons–Wadsworth reagent, tetraethyl methylene-1,1-bisphosphonate in DMF/LiOH (aq) solution led exclusively to a meso form of nitrogen-containing tetraphosphonates (NTPs) (bis(α-aminobisphosphonates)). Next, hydrolysis of the ester moieties of the tetraphosphonate products yielded the corresponding tetraphosphonic acids, which treated with MeOH/KOH (aq, 20%) to give the respective NTP-tetrapotassium salts. Prior to synthesis, the suggested structures (and others) were applied to the computer-assisted molecular modeling, PASS program to investigate their prospective biological properties. Cytotoxic properties were later evaluated against five malignant melanoma cell lines that originated from different categories of malignant melanoma primary stage (I/II), histologically advanced stage (III/IV), and metastasized malignancy. Almost all tested compounds showed antitumor activity though on different levels. Three NTP salts were found to have activity in the range of GI50 0.650–5.73 μM vs. control reference GI50: 1.745–6.50 μM. Structure activity relationship is also discussed.
Graphical abstract




Similar content being viewed by others
References
Li J, Xu L-Z, He K-L, Guo W-J, Zheng Y-H, Xia P, Chen Y (2001) Breast Cancer Res 3:253
Engel J, Eckel R, Kerr J, Schmidt M, Furstenberger G, Richter R, Sauer H, Senn HJ (2001) Ital J Anat Embryol 106:59
Uchiyama-Kokubu N, Watanabe T (2001) Anticancer Drugs 12:769
Cree A, Knight L, Di Nicolantonio LF, Sharma S, Gulliford T (2002) Curr Opin Investig Drugs 3:634
Guo H, Miao Y (2013) Bioorg Med Chem Lett 23:2319
Ascierto PA, Kikwood JM, Marincola FM, Palmieri G (2011) J Skin Cancer 2011 (article ID 710697)
Divito SJ, Ferris LK (2010) Melanoma 20:450
Rondeau JM, Bitsch F, Bourgier E, Geiser M, Hemmig R, Kroemer M, Lehmann S, Ramage P, Rieffel S, Strauss A, Green JR, Jahnke W (2006) Chem Med Chem 1:267
Cancer Research UK. http://www.cancerresearchuk.org/about-melanoma/bowel-cancer/stages-grades/report2014-1
Balakrishna A, Reddy MVN, Rao PV, Kumar MA, Kumar BS, Nayak SK, Reddy CS (2011) Eur J Med Chem 46:1798
Hughes DE, Wright KR, Uy HL, Sasaki A, Yoneda T, Roodman GD, Mundy GR, Boyce BF (1995) J Bone Miner Res 10:1478
Reszka AA, Rodan GA (2003) Curr Osteoporos Rep 1:45
Aznar S, Lacal JC (2001) Cancer Lett 165:1
van Beek E, Pieterman E, Cohen L, Lowik C, Papapoulos S (1999) Biochem Biophys Res Commun 264:108
Abdou WM, Barghash RF, Bekheit MS, Geronikaki A (2016) Chem Select 1:3797
Abdou WM, Shaddy AA, Khidre RE, Awad GEA (2016) J Heterocycl Chem 53:524
Shaddy AA, Kamel AA, Abdou WM (2013) Synth Commun 43:236
Abdou WM, Barghash RF, Sediek AA (2012) Eur J Med Chem 57:362
Abdou WM, Kamel AA, Khidre RE, Geronikaki A, Ekonomopoulou MT (2012) Chem Biol Drug Des 79:719
Abdou WM, Khidre RE, Kamel AA (2012) Arch Pharm Chem Life Sci 345:123
Meta-analysis finds benefits of adjuvant bisphosphonates for postmenopausal breast cancer. Cancer Research Institute, NY, USA, Report September 9, 2015
Cheng F, Oldfield E (2004) J Med Chem 47:5149
Coleman RE (2001) Cancer Treat Rev 27:165
Fleisch H (2002) Breast Cancer Res 4:30
Shipman CM, Rogers MJ, Apperley JF, Russell RG, Croucher PI (1997) Br J Haematol 98:665
Shipman CM, Croucher PI, Russell RG, Helfrich MH, Rogers MJ (1998) Cancer Res 58:5294
Riebeling C, Forsea AM, Raisova M, Orfanos CE, Geilen CC (2002) Br J Cancer 87:366
Forsea A-M, Müller C, Riebeling C, Orfanos CE, Geilen CC (2004) Br J Cancer 91:803
Poroikov V, Filimonov D et al (1992–2014) PASS 14 Standard-prediction of activity spectra for substances. http://www.pharmaexpert.ru
Poroikov V, Filimonov D (2005) PASS: prediction of biological activity spectra for substances. In: Helma C (ed) Predictive toxicology. Taylor & Francis, London, p 459
Abdel-Fatah TM, McArdle SE, Agarwal D, Moseley PM, Green AR, Ball GR, Pockley AG, Ellis IO, Rees RC, Chan SY (2016) Clin Cancer Res 22:905
Goel RK, Singh A, Naidu PS, Mahajan MP, Kulkarni SK (2005) J Pharm Pharm Sci 8:182
Singh G, Bansal Y, Bansal G, Goel RK (2014) Med Chem 10:418
Poroikov VV, Filimonov DA (2002) J Comput Aided Mol Des 16:819
Das S, Das VK, Saikia L, Thakur AJ (2012) Green Chem Lett Rev 5:457
Kiviranta PH, Leppönen J, Kyrylenko S, Salo HS, Lahtela-Kakkonen M, Tervo AJ, Wittekindt C, Suuronen T, Kuusisto E, Jörvinen T, Salminen A, Poso A, Wallén EAA (2006) J Med Chem 49:7907
Sierra MA, Gómez-Gallego M, Alcázar R, Lucena JJ, Yuntab F, Garcia-Marcob S (2004) Dalton Trans (21):3741
Pudovik AN, Pudovik MA (1966) Zh Obshch Khim 36:1467
Manouni DE, Benech JM, Benramdane M, Lecouvey M, Leger G, Leroux Y (1999) Phosphorus. Sulfur Silicon Relat Elem 147:81
Kanaan M, Burgada R (1988) Phosphorus Sulfur Relat Elem 37:217
Vollhardt KPC, Shore N (2007) Organic Chemistry, 5th edn. Freeman WH, New York
Shore N (2007) Study guide and solutions manual for organic chemistry, 5th edn. Freeman WH, New York
Kraicheva I, Finocchiaro P, Failla S (2007) Phosphorus. Sulfur Silicon Relat Elem 182:57
Lewkowska J, Lewkowska ES (2006) Phosphorus. Sulfur Silicon Relat Elem 181:1323
Lewkowska J (2005) Phosphorus. Sulfur Silicon Relat Elem 180:179
Barycki J, Garncarz R, Milewska M, Tyka R (1995) Phosphorus. Sulfur Silicon Relat Elem 105:117
Nugent RA, Murphy M, Schlachter ST, Dunn CJ, Smith RJ, Staite ND, Galinet LA, Shields SK, Aspar DG, Richard KA, Rohloff NA (1993) J Med Chem 36:134
Ebetino FHM, Francis D, Rogers MJ, Russell RGG (1998) Rev Contemp Pharmacother 9:233
Grever MR, Schepartz SA, Chabner BA (1992) Semin Oncol 19:622
Boyd MR, Paull KD (1995) Drug Dev Res 34:91
Skehan P, Storeng R, Scudiero D, Monks A, Mahon JMC, Vistica D, Warren JT, Bokesh H, Kenney S, Boyd MR (1990) J Natl Cancer Inst 82:1107
Karber C (1931) Naunyn-Schmiedebergs Arch Exp Pathol Pharmakol 162:480
Girgis AS, Mishriky N, Ellithey M, Hosni HM, Farag H (2007) Bioorg Med Chem 15:2403
Acknowledgements
The authors would like to thank the National Research Centre, Dokki, Cairo, Egypt (project # 10010340) for the financial support of the present work. They also are grateful to Cancer Research Institute, NY, USA, for handling the antitumor properties.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bekheit, M.S., Barghash, R.F. & Abdou, W.M. Computer-aided design, synthesis, and biological studies of anticological nitrogen-containing tetraphosphonic acids against melanoma. Monatsh Chem 149, 1481–1491 (2018). https://doi.org/10.1007/s00706-018-2196-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-018-2196-9