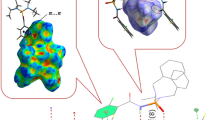
Abstract
In the present paper, crystal structures and Hirshfeld surface analyses of two new phosphoric triamides [2,3,6-F3–C6H2C(O)NH]P(O)(X)2 (X=N(CH3)C6H11 and N(C2H5)2) and an improved model of [OH8C4N]3P(O) are investigated. Moreover, the semi-classical density sums (PIXEL) method, which enables the calculation of interaction energies for molecule–molecule pairs, and AIM calculations were used to evaluate intermolecular forces in the studied compounds. The previously reported structure [2,6-F2-C6H3C(O)NH]P(O)[NHC(CH3)3]2 with a [C(O)NH]P(O)[NH(C)]2 segment, which is different than the [C(O)NH]P(O)[N(C)(C)]2 segment in structures [2,3,6-F3–C6H2C(O)NH]P(O)(X)2, is compared to those of the newly determined structures. The Hirshfeld surface method shows that the crystal cohesions of structures [2,3,6-F3–C6H2C(O)NH]P(O)(X)2 are established via H···H, O···H/H···O, C···H/H···C, and F···H/H···F contacts, while for [OH8C4N]3P(O), H···H and O···H/H···O are the dominant contacts. From PIXEL and AIM calculations and the decomposition of the interaction energies for different molecular pairs, it is shown that the donor and acceptor capability of the atoms involved in an interaction introduces the nature and strength of that interaction. The more acidic NCP–H unit in the C(O)NHP(O) segment (compared to the NP–H unit in the P(O)[NH(C)]2 segment) and the higher H-atom acceptor group P=O (compared to C=O) in the studied structures form the strongest NCP–H···O=P intermolecular hydrogen bond.
Graphical abstract













Similar content being viewed by others
References
Hoon Kwon C, Young Moon K, Baturay N, Shirota FN (1991) J Med Chem 34:588
Jain M, Fan J, Baturay NZ, Kwon C-H (2004) J Med Chem 47:3843
Hu L, Yu C, Jiang Y, Han J, Li Z, Browne P, Race PR, Knox RJ, Searle PF, Hyde EI (2003) J Med Chem 46:4818
Quintero L, Sánchez-Vazquez M, Cruz-Gregorio S, Sartillo-Piscil F (2010) J Org Chem 75:5852
Font M, Domínguez M-J, Sanmartín C, Palop JA, San-Francisco S, Urrutia O, Houdusse F, García-Mina JM (2008) J Agric Food Chem 56:8451
Domínguez MJ, Sanmartín C, Font M, Palop JA, San Francisco S, Urrutia O, Houdusse F, García-Mina JM (2008) J Agric Food Chem 56:3721
Nakashima D, Yamamoto H (2006) J Am Chem Soc 128:9626
Nishikawa Y, Nakano S, Tahira Y, Terazawa K, Yamazaki K, Kitamura CH, Hara O (2016) Org Lett 18:2004
Pourayoubi M, Toghraee M, Zhu J, Dušek M, Bereciartua PJ, Eigner V (2014) CrystEngComm 16:10870
Palatinus L, Brázda P, Boullay P, Perez O, Klementová M, Petit S, Eigner V, Zaarour M, Mintova S (2017) Science 355:166
Capelli SC, Bürgi H-B, Dittrich B, Grabowsky S, Jayatilaka D (2014) IUCrJ 1:361
Woińska M, Grabowsky S, Dominiak PM, Woźniak K, Jayatilaka D (2016) Sci Adv 2:e1600192
McKinnon JJ, Mitchell AS, Spackman MA (1998) Chem Eur J 4:2136
Spackman MA, McKinnon JJ (2002) CrystEngComm 4:378
Dunitz JD, Gavezzotti A, Rizzato S (2014) Cryst Growth Des 14:357
Shukla R, Shripanavar C, Chopra D, Bubbly SG, Gudennavar SB (2015) Struct Chem Cryst Comm 1:1
Gavezzotti A (2011) New J Chem 35:1360
Pourayoubi M, Tarahhomi A, Rheingold AL, Golen JA (2010) Acta Cryst E66:o3159
Romming C, Songstad J (1982) Acta Chem Scand A 36:665
Tarahhomi A, Pourayoubi M, Golen JA, Zargaran P, Elahi B, Rheingold AL, Leyva Ramírez MA, Mancilla Percino T (2013) Acta Cryst B69:260
Tarahhomi A, Pourayoubi M, Rheingold AL, Golen JA (2011) Struct Chem 22:201
Pourayoubi M, Tarahhomi A, Saneei A, Rheingold AL, Golen JA (2011) Acta Cryst C67:o265
Pourayoubi M, Toghraee M, Divjakovic V, van der Lee A, Mancilla Percino T, Leyva Ramírez MA, Saneei A (2013) Acta Cryst B69:184
Mazur L, Koziol AE, Jarzembska KN, Paprocka R, Modzelewska-Banachiewicz B (2017) Cryst Growth Des 17:2104
Martin AD, Britton J, Easun TL, Blake AJ, Lewis W, Schröder M (2015) Cryst Growth Des 15:1697
Agilent CrysAlis PRO (2011) Agilent technologies. Yarnton, Oxfordshire
Palatinus L, Chapuis G (2007) J Appl Cryst 40:786
Betteridge PW, Carruthers JR, Cooper RI, Prout K, Watkin DJ (2003) J Appl Cryst 36:1487
Cooper RI, Thompson AL, Watkin DJ (2010) J Appl Cryst 43:1100
Spek AL (2009) Acta Cryst D65:148
Macrae CF, Bruno IJ, Chisholm JA, Edgington PR, McCabe P, Pidcock E, Rodriguez-Monge L, Taylor R, van de Streek J, Wood PA (2008) J Appl Cryst 41:466
de la Flor G, Orobengoa D, Tasci E, Perez-Mato JM, Aroyo MI (2016) J Appl Cryst 49:653
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery Jr JA, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2009) Gaussian 09, Revision B.04. Gaussian, Inc, Pittsburgh, PA
Dunitz JD, Gavezzotti A (2012) Cryst Growth Des 12:5873
Feynman RP (1989) The Feynman lectures on physics, vol 2. Addison-Wesley, Reading
London F (1937) Trans Faraday Soc 33:8
Kauzmann W (1957) Quantum chemistry, an introduction. Academic Press, New York, p 305
Bader RFW (1990) Atoms in molecules, a quantum theory. Oxford University Press, New York
Turner MJ, McKinnon JJ, Wolff SK, Grimwood DJ, Spackman PR, Jayatilaka D, Spackman MA (2017) CrystalExplorer17. University of Western Australia
McKinnon JJ, Spackman MA, Mitchell AS (2004) Acta Cryst B60:627
McKinnon JJ, Jayatilaka D, Spackman MA (2007) Chem Commun 3814
Spackman MA, Jayatilaka D (2009) Cryst EngComm 11:19
Fabbiani FPA, Leech CK, Shankland K, Johnston A, Fernandes P, Florence AJ, Shankland N (2007) Acta Cryst C63:o659
Acknowledgements
Support of this investigation by Semnan University is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tarahhomi, A., van der Lee, A. Synthesis and crystal structures of new phosphoric triamides: study of intermolecular interactions by semi-empirical calculations and Hirshfeld surface analysis. Monatsh Chem 149, 1759–1776 (2018). https://doi.org/10.1007/s00706-018-2186-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-018-2186-y