Abstract
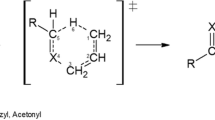
The thermal decomposition kinetics of allyl methyl amine, allyl methyl ether, and allyl methyl sulfide in the gas phase has been studied theoretically using the M06-2x/aug-cc-pVTZ quantum chemical approach. The observed activation parameters are consistent with a concerted unimolecular mechanism involving a non-planar cyclic six-membered transition state. Based on the optimized ground state geometries, a natural bond orbital analysis of donor–acceptor interactions reveals that the stabilization energies corresponding to the electronic delocalization from the lone-pair (LP) non-bonding orbitals on the heteroatom to the neighboring \(\sigma_{{{\text{C2}} - {\text{C3}}}}^{*}\) antibonding orbitals decrease from allyl methyl amine to allyl methyl sulfide. This delocalization fairly explains the increase of occupancies of LP orbitals on the heteroatom from allyl methyl sulfide to allyl methyl amine. The results also suggest that the kinetics of the thermolysis of the studied compounds are dominated by \({\text{LP}}\, \to \,\sigma^{*}\) electronic delocalization effects. Analysis of bond order, bond indices, and synchronicity parameters demonstrates that these reactions proceed through a concerted and slightly asynchronous mechanism.
Graphical abstract






Similar content being viewed by others
References
Vitins P, Egger KW (1974) J Chem Soc Perkin Trans 2:1289
Egger KW, Vitins P (1974) Int J Chem Kinet 6:429
Kwart H, Sarner SF, Slutsky J (1973) J Am Chem Soc 95:5234
Martin G, Ropero M, Avila R (1982) Phosphorus Sulfur Relat Elem 13:213
Eyring H (1935) J Chem Phys 3:107
Johnston HS (1966) Gas phase reaction rate theory. Roland Press, New York
Laidler KJ (1969) Theories of chemical reaction rates. McGraw-Hill, New York
Weston RE, Schwartz HA (1972) Chemical kinetics. Prentice-Hall, New York
Rapp D (1972) Statistical mechanics. Holt, Rinehart, and Winston, New York
Nikitin EE (1974) Theory of elementary atomic and molecular processes in gases. Clarendon Press, Oxford
Smith IWM (1980) Kinetics and dynamics of elementary gas reactions. Butterworths, London
Reed AE, Curtiss LA, Weinhold F (1988) Chem Rev 88:899
Zhao Y, Truhlar DG (2008) Theor Chem Acc 120:215
Dunning TH (1989) J Chem Phys 90:1007
Robinson PJ, Holbrook KA (1972) Unimolecular reactions. Wiley, New York
Steinfeld JI, Francisco JS, Hase WL (1999) Chemical kinetics and dynamics. Prentice-Hall, Englewood Cliffs
Eyring H, Lin SH, Lin SM (1980) Basic chemical kinetics. Wiley, New York
Reed AE, Weinstock RB, Weinhold F (1985) J Chem Phys 83:735
Badenhoop JK, Weinhold F (1999) Int J Quantum Chem 72:269
Hammond GS (1953) J Am Chem Soc 77:334
Agmon N, Levine RD (1977) Chem Phys Lett 52:197
Bickelhaupt FM, Houk KN (2017) Angew Chem Int Ed 56:10070
Ess DH, Houk KN (2007) J Am Chem Soc 129:10646
Legault CY, Garcia Y, Merlic CA, Houk KN (2007) J Am Chem Soc 129:12664
Ess DH, Houk KN (2008) J Am Chem Soc 130:10187
Hayden AE, Houk KN (2009) J Am Chem Soc 131:4084
Schoenebeck F, Ess DH, Jones GO, Houk KN (2009) J Am Chem Soc 131:8121
van Zeist W-J, Bickelhaupt FM (2010) Org Biomol Chem 8:3118
Fernández I, Cossío FP, Bickelhaupt FM (2011) J Org Chem 76:2310
Fernández I, Bickelhaupt FM (2012) J Comput Chem 33:509
Fernández I, Bickelhaupt FM, Cossío FP (2012) Chem Eur J 18:12395
Fernández I, Cossío FP, Sierra MA (2009) Chem Rev 109:6687
Fernández I, Bickelhaupt FM, Cossío FP (2014) Chem Eur J 20:10791
Fernández I, Bickelhaupt FM (2014) Chem Soc Rev 43:4953
Ziegler T, Rauk A (1977) Theor Chim Acta 46:1
Ziegler T, Rauk A (1979) Inorg Chem 18:1558
Bickelhaupt FM, Ziegler T, von Rague Schleyer P (1995) Organometallics 14:2288
Lendvay G (1989) J Phys Chem 93:4422
Wiberg KB (1968) Tetrahedron 24:1083
Glendening ED, Reed AE, Carpenter JE, Weinhold F (1998) NBO Version 3.1. Theoretical Chemistry Institute and Department of Chemistry, University of Wisconsin, Madison
Moyano A, Periclas MA, Valenti E (1989) J Org Chem 54:573
Rosas F, Dominguez RM, Tosta M, Mora JR, Marquez E, Cordova T, Chuchani G (2010) J Phys Org Chem 23:743
Knippenberg S, Bohnwagner MV, Harbach PH, Dreuw A (2015) J Phys Chem A 119:1323
Chai JD, Head-Gordon M (2008) Phys Chem Chem Phys 10:6615
Shiroudi A, Deleuze MS (2015) J Mol Model 21:301
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, Revision A.1. Gaussian, Inc, Wallingford
Parr RG, Wang W (1989) Density functional theory of atoms and molecules. Oxford University Press, New York
Gonzalez C, Schlegel HB (1989) J Chem Phys 90:2154
Gonzalez C, Schlegel HB (1990) J Phys Chem 94:5523
Chang R (2005) Physical chemistry for the biosciences. University Science Books, Sausalito
Moore JW, Pearson RG (1981) Kinetics and mechanism—the study of homogeneous chemical reactions. Wiley, New York
Carstensen HH, Dean AM, Deutschmann O (2007) Proc Combust Inst 31:149
Eckart C (1930) Phys Rev 35:1303
Johnson HS, Heicklen J (1962) J Phys Chem 66:532
Forst W (1973) Theory of unimolecular reactions. Academic Press, New York
Beyer T, Swinehart DF (1973) Commun Assoc Comput Mach 16:379
Stein SE, Rabinovitch BS (1973) J Chem Phys 58:2438
Canneaux S, Bohr F, Henon E (2014) J Comput Chem 35:82
Mourits FM, Rummens HA (1977) Can J Chem 55:3007
Kee RJ, Rupley FM, Miller JA, Coltrin ME, Grcar JF, Meeks E, Moffat HK, Lutz AE, Dixon-Lewis G, Smooke MD, Warnatz J, Evans GH, Larson RS, Mitchell RE, Petzold LR, Reynolds WC, Caracotsios M, Stewart WE, Glarborg P, Wang C, McLellan CL, Adigun O, Houf WG, Chou CP, Miller SF, Ho P, Young PD, Young DJ, Hodgson DW, Petrova MV, Puduppakkam KV (2010) Chemkin, Reaction design. San Diego, California
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Oliaey, A.R., Shiroudi, A., Zahedi, E. et al. Theoretical study on the elimination kinetics in the gas phase of allyl methyl compounds. Monatsh Chem 149, 1389–1400 (2018). https://doi.org/10.1007/s00706-018-2184-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-018-2184-0