Abstract
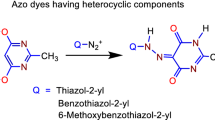
A series of azo naphthalimide dyes were prepared by coupling N,N-diethyl-m-toluidine with three heterocyclic amines as the diazo components. The structures of the nine prepared azo dyes were fully characterized by Fourier transform infrared, differential scanning calorimeter, thin-layer chromatography, proton and carbon nuclear magnetic resonance, mass spectrometry, elemental analysis (CHNS), and UV–Vis spectroscopic techniques. UV–Vis studies represented that all dyes have molar extinction coefficients between 30,000 and 61,000 M−1 cm−1 and have maximum wavelengths between 545 and 558 nm in DMF solution. The newly synthesized dyes were screened for their potential antimicrobial activities against Gram-positive, Gram-negative bacteria and fungi using the cup plate and broth microdilution methods. The results of antibacterial and antifungal activities exhibited significant inhibitory effect against the test microorganisms in vitro.
Graphical abstract




Similar content being viewed by others
References
Zhang YY, Zhou CH (2011) Bioorg Med Chem Lett 21:4349
Sk UH, Gowda ASP, Crampsie MA, Yun JK, Spratt TE, Amin S, Sharma AK (2011) Eur J Med Chem 46:3331
Hariprakasha HK, Kosakowska Cholody T, Meyer C, Cholody WM, Stinson SF, Tarasova NI, Michejda CJ (2007) J Med Chem 50:5557
Konstantinova TN, Miladinova PM (2009) J Appl Polym Sci 111:1991
Damu Guri LV, Wang QP, Zhang ZH, Zhang YY, Lv JS, Zhou CH (2013) Sci China Chem 56:952
Shaki H, Gharanjig K, Khosravi A (2015) Indian J Fibre Text 40:425
Lv JS, Peng XM, Kishore B, Zhou CH (2014) Bioorg Med Chem Lett 24:308
Horton JK, Wilson SH (2013) Mol Cancer Res 11:13
Grabchev I, Staneva D, Betcheva R (2012) Curr Med Chem 19:4976
Jeong Y, Yoon J (2012) Inorg Chim Acta 381:2
Banerjee S, Veale EB, Phelan CM, Murphy SA, Tocci GM, Gillespie LJ, Frimannsson DO, Kelly JM, Gunnlaugsson T (2013) Chem Soc Rev 42:1601
Roger JE, Abraham B, Rostkowski A, Kelly LA (2001) Photochem Photobiol 74:521
Kilpin J, Clavel CM, Edafe F, Dyson PJ (2012) Organometallics 31:7031
Muth M, Hoerr V, Glaser M, Ponte-Sucre A, Moll H, Stich A, Holzgrabe U (2007) Bioorg Med Chem Lett 17:1590
Zhang YY, Mi JL, Zhou CH, Zhou XD (2011) Eur J Med Chem 46:4391
Abuo-Rahma GEDAA, Sarhan HA, Gad GFM (2009) Bioorg Med Chem 17:3879
Duke RM, Veale EB, Pfeffer FM, Krugerc PE, Gunnlaugsson T (2010) Chem Soc Rev 39:3936
Brana MF, Castellano JM, Roldan CM, Santos A, Vazquez D, Jimenez A (1980) Cancer Chemother Pharm 4:61
Stevenson KA, Yen SF, Yang NC, Boykin DW, Wilson WD (1984) J Med Chem 27:1677
Tao ZF, Qian XH, Wei D (1996) Dyes Pigm 31:245
Li ZG, Yang Q, Qian XH (2005) Bioorg Med Chem 13:4864
Chen Z, Liang X, Zhang H, Xie H, Liu J, Xu Y, Zhu W, Wang Y, Wang X, Tan S, Kuang D, Qian XH (2010) J Med Chem 53:2589
Kamal A, Satyanarayana M, Devaiah V, Rohini V, Yadav JS, Mullickm B, Nagaraja V (2006) Lett Drug Des Discov 3:494
Peters AT, Bide MJ (1986) Dyes Pigm 7:237
Shaki H, Gharanjig K, Rouhani S, Khosravi A, Fakhar J (2012) Color Technol 128:270
Shaki H, Gharanjig K, Rouhani S, Khosravi A (2010) J Photochem Photobiol A 216:44
Dodangeh M, Gharanjig K, Arami M (2014) IEEE Sensor J 14:2889
Safabakhsh B, Khosravi A, Gharanjig K, Kowsari E, Khorassani M, Tafaghodi S (2012) Color Technol 128:218
Shaki H, Gharanjig K, Khosravi A (2015) Biotechnol Progr 31:1086
Sharshira EM, Hamada NMM (2012) Am J Org Chem 2:69
Luo YL, Baathulaa K, Kannekanti VK, Zhou CH, Cai GX (2015) Sci China Chem 58:483
Moanta A, Ionescu C, Dragoi M, Tutunaru B, Rotaru P (2015) J Therm Anal Calorim 120:1151
Salmon AJ, Williams ML, Wu QK, Morizzi J, Gregg D, Charman SA, Vullo D, Supuran CT, Poulson SA (2012) J Med Chem 55:5506
Sayed AZ, El-Gaby MSA (2001) Color Technol 117:293
Shaki H, Khosravi A, Gharanjig K, Mahboubi A (2016) Mater Technol 31:322
Wojciechowski K (1993) Dyes Pigm 22:117
Kose M, Kurtoglu N, Gumussu O, Tutak M, McKee V, Karakas D, Kurtoglu M (2013) J Mol Struct 1053:89
Ebead YH (2011) Dyes Pigm 92:705
Velasco MI, Kinen CO, De Rossi RH, Rossi LI (2011) Dyes Pigm 90:259
Wojciechowski K (1990) Dyes Pigm 12:273
Wojcicchowski K (1997) Dyes Pigm 33:149
Griffiths J (1984) Developments in the chemistry and technology of organic dyes. Blackwell Scientific Publications, London
Liu S, Ma J, Zhao D (2007) Dyes Pigm 75:255
Keerthi Kumar CT, Keshavayya J, Rajesh T, Peethambar SK, Shoukat Ali RA (2013) Chem Sci Trans 2:1346
Fazly Bazzaz BS, Khajehkaramadin M, Shokooheizadeh HR (2005) Iran J Pharm Res 4:87
Clinical and Laboratory Standards Institute (2006) Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 7th edn, p 14
Acknowledgments
The author is grateful to the Golestan University. This work was supported by the Golestan University (project No. 961527).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shaki, H. Novel monoazo disperse and cationic dyes: preparation, structure investigation, study of spectroscopic, antibacterial and antifungal potential. Monatsh Chem 149, 1149–1160 (2018). https://doi.org/10.1007/s00706-017-2130-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-017-2130-6