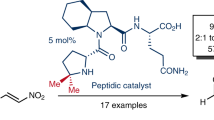
Abstract
Stereoselective Michael addition of enolizable carbonyl compounds to a furane-derived nitroalkene was catalyzed by di- and tripeptide organocatalysts. The most competent catalysts were tripeptides possessing Pro–Pro–Glu structure. With aldehydes, Michael adducts were obtained in high yields and with medium-to-high diastereo- (up to 13:1 d.r.) and enantiomeric purities (up to 99% ee). The reaction was less stereoselective with cyclic ketones than with aldehydes.
Graphical abstract




Similar content being viewed by others
References
Joule JA, Mills K (2010) Heterocyclic chemistry, 5th edn. Wiley-Blackwell, Chichester
Corey EJ, Czakó B, Kürti L (2007) Molecules and medicine. Wiley, Hoboken
Albrecht L, Ransborg LK, Jørgensen KA (2012) Catal Sci Technol 2:1089
Vetica F, Chauhan P, Dochain S, Enders D (2017) Chem Soc Rev 46:1661
Sethuraman I, Jose CM, Subbu P (2013) Curr Org Chem 17:2038
Zhang Y, Wang W (2012) Catal Sci Technol 2:42
Vicario JL, Badia D, Carrillo L (2007) Synthesis 2007:2065
Tsogoeva SB (2007) Eur J Org Chem 2007:1701
Miller SJ (2004) Acc Chem Res 37:601
Davie EAC, Mennen SM, Xu Y, Miller SJ (2007) Chem Rev 107:5759
Wennemers H (2011) Chem Commun 47:12036
Lewandowski B, Wennemers H (2014) Curr Opin Chem Biol 22:40
Shugrue CR, Miller SJ (2017) Chem Rev 117:11894
Miller SJ, Copeland GT, Papaioannou N, Horstmann TE, Ruel EM (1998) J Am Chem Soc 120:1629
Jordan PA, Kayser-Bricker KJ, Miller SJ (2010) Proc Natl Acad Sci USA 107:20620
Han S, Miller SJ (2013) J Am Chem Soc 135:12414
Fiori KW, Puchlopek ALA, Miller SJ (2009) Nat Chem 1:630
Krattiger P, Kovasy R, Revell JD, Ivan S, Wennemers H (2005) Org Lett 7:1101
Córdova A, Zou W, Dziedzic P, Ibrahem I, Reyes E, Xu Y (2006) Chem Eur J 12:5383
Dziedzic P, Zou W, Hafren J, Cordova A (2006) Org Biomol Chem 4:38
Revell JD, Wennemers H (2008) Adv Synth Catal 350:1046
Yan J, Wang L (2009) Chirality 21:413
Bayat S, Tejo BA, Salleh AB, Abdmalek E, Normi YM, Rahman MBA (2013) Chirality 25:726
Psarra A, Kokotos CG, Moutevelis-Minakakis P (2014) Tetrahedron 70:608
Triandafillidi I, Bisticha A, Voutyritsa E, Galiatsatou G, Kokotos CG (2015) Tetrahedron 71:932
Vega-Peñaloza A, Sánchez-Antonio O, Ávila-Ortiz CG, Escudero-Casao M, Juaristi E (2014) Asian J Org Chem 3:487
Bisticha A, Triandafillidi I, Kokotos CG (2015) Tetrahedron Asymmetry 26:102
Revell JD, Gantenbein D, Krattiger P, Wennemers H (2006) Biopolymers (Pept Sci) 84:105
Xu Y, Zou W, Sundén H, Ibrahem I, Córdova A (2006) Adv Synth Catal 348:418
Wiesner M, Revell JD, Wennemers H (2008) Angew Chem Int Ed 47:1871
Wiesner M, Revell JD, Tonazzi S, Wennemers H (2008) J Am Chem Soc 130:5610
Wiesner M, Wennemers H (2010) Synthesis 2010:1568
Duschmalé J, Wennemers H (2012) Chem Eur J 18:1111
Bächle F, Duschmalé J, Ebner C, Pfaltz A, Wennemers H (2013) Angew Chem Int Ed 52:12619
Duschmale J, Kohrt S, Wennemers H (2014) Chem Commun 50:8109
Kastl R, Wennemers H (2013) Angew Chem Int Ed 52:7228
Arakawa Y, Wiesner M, Wennemers H (2011) Adv Synth Catal 353:1201
Arakawa Y, Wennemers H (2013) Chemsuschem 6:242
Grünenfelder CE, Kisunzu JK, Wennemers H (2016) Angew Chem Int Ed 55:8571
Akagawa K, Satou J, Kudo K (2016) J Org Chem 81:9396
Akagawa K, Iwasaki Y, Kudo K (2016) Eur J Org Chem 2016:4460
Durini M, Sahr FA, Kuhn M, Civera M, Gennari C, Piarulli U (2011) Eur J Org Chem 2011:5599
Borges-González J, Feher-Voelger A, Crisóstomo FP, Morales EQ, Martín T (2017) Adv Synth Catal 359:576
Cao D, Fang G, Zhang J, Wang H, Zheng C, Zhao G (2016) J Org Chem 81:9973
Akagawa K, Kudo K (2017) Acc Chem Res 50:2429
Metrano AJ, Abascal NC, Mercado BQ, Paulson EK, Hurtley AE, Miller SJ (2017) J Am Chem Soc 139:492
Gao S, Tu YQ, Hu X, Wang S, Hua R, Jiang Y, Zhao Y, Fan X, Zhang S (2006) Org Lett 8:2373
Pansare SV, Pandya K (2006) J Am Chem Soc 128:9624
Benoiton NL (2006) Chemistry of peptide synthesis. Taylor & Francis, Boca Raton
Lu D, Gong Y, Wang W (2010) Adv Synth Catal 352:644
Chen F, Huang S, Zhang H, Liu F, Peng Y (2008) Tetrahedron 64:9585
Hernández JG, García-López V, Juaristi E (2012) Tetrahedron 68:92
de Boer JW, Browne WR, Harutyunyan SR, Bini L, Tiemersma-Wegman TD, Alsters PL, Hage R, Feringa BL (2008) Chem Commun:3747
Tsunematsu H, Isobe R, Hanazono H, Soeda Y, Inagaki M, Ito N, Higuchi R, Yamamoto M (1999) Chem Pharm Bull 47:1040
Hiroshi F, Hidenori K, Norio I, Ichizo M, Hideo O (1983) Bull Chem Soc Jpn 56:766
Zhang A, Guo Y (2008) Chem Eur J 14:8939
Yu H, Liu M, Han S (2014) Tetrahedron 70:8380
Wang Y, Lin J, Wei K (2014) Tetrahedron Asymmetry 25:1599
Zheng Z, Perkins BL, Ni B (2010) J Am Chem Soc 132:50
Nugent TC, Bibi A, Sadiq A, Shoaib M, Umar MN, Tehrani FN (2012) Org Biomol Chem 10:9287
Acknowledgements
This work was supported by the Slovak Research and Development Agency under the contract no. APVV-15-0039.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Poláčková, V., Čmelová, P., Górová, R. et al. Peptide-catalyzed stereoselective Michael addition of aldehydes and ketones to heterocyclic nitroalkenes. Monatsh Chem 149, 729–736 (2018). https://doi.org/10.1007/s00706-017-2126-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-017-2126-2